Author:
Kai Knudsen
Updated:
27 August, 2025
Here, the principles of ventilator treatment for acute lung failure and severe lung failure with ARDS are described. Various ventilator settings and the treatment of severe respiratory failure are discussed. The principles of treating severe pulmonary infection are also covered. Different ventilator settings (modes) in various types of ventilators are presented, as well as CPAP, BiPAP, and High-Flow Nasal Cannula (Optiflow). Weaning from the ventilator and lung recruitment are described here.
- Ventilator Treatment
- Ventilator Treatment in Severe Respiratory Failure
- Ventilation Care Recommendations
- Ventilator treatment in ARDS
- ARDS
- Fine Tuning in the Ventilator
- Pressure Supported Ventilation (PSV)
- Weaning from the ventilator
- Pressure Supported Ventilation (PS)
- Weaning from Ventilator – Practical Advice
- Lung Recruitment
- Extubation
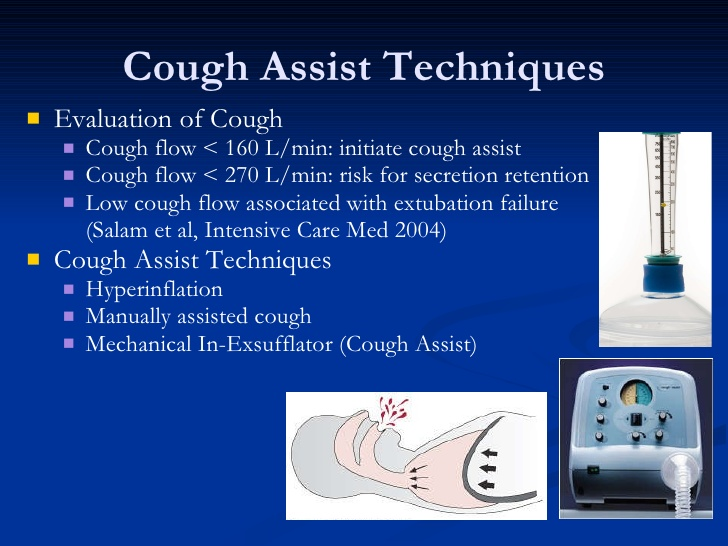
- Coughing – During Ventilator Treatment
- HFNC – High-Flow Nasal Cannula – Optiflow
- CPAP – Continuous Positive Airway Pressure
- PEEP – Positive End-Expiratory Pressure
- ASV – Adaptive Support Ventilation
- BiPAP – Biphasic Positive Airway Pressure
- BiPAP-assist (with ASB)
- (S)CMV+
- APRV – Airway Pressure Release Ventilation
- DuoPAP Breathing Pattern
- IPPV – Intermittent Positive Pressure Ventilation
- MMV – Mandatory Minute Ventilation
- PCV+
- SIMV – Synchronized Intermittent Mandatory Ventilation
- SIMV ASB (Assisted Spontaneous Breathing)
- PSIMV+
- VCV – Volume Controlled Ventilation
- PCV – Pressure Controlled Ventilation
- PSV – Pressure Supported Ventilation
- VSV – Volume Supported Ventilation
- VCPR – Volume Controlled Pressure Regulation
- Automode VCPS – VS
- Automode VC – VS
- Automode PC + PS
- NAVA – Neurally Adjusted Ventilatory Assist
- Ventilator Treatment for Children
- Maquet/Siemens Ventilators (Getinge)
- Aisys (ADU)(Datex-Ohmeda – GE Healthcare)
- Evita XL, Evita 4, Pulmovista (Dräger Ventilators)
Ventilator Treatment
Patients who, due to lung disease or other damage to the lungs, cannot breathe adequately can be treated with a ventilator that assists with lung ventilation. The ventilator is also referred to as a respirator. Breathing occurs invasively through a closed tube system with regulated and controlled pressure and airflow in the airways. The ventilator delivers a preset tidal volume or minute volume during a preset inspiration time and at a preset breathing frequency. The patient is typically orally intubated but may also be nasally intubated or connected via a tracheal cannula in a tracheostomy.
Normal spontaneous breathing physiologically occurs through negative pressure in the airways, where air is drawn into the lungs by the respiratory muscles. In ventilator-generated ventilation, air is blown into the lungs under positive pressure. The ventilator’s compressed air is mechanically generated by a compressor, usually located at the back of the respiratory system. A system of precision valves then controls the gas pressure and flow to the patient, which is regulated by the user digitally or analogically in different breathing methods. The varying breathing methods are often referred to as “ventilator modes” or “breathing modes” in the ventilator. A more common term is “ventilator settings” or “breathing method in the ventilator.”
The most common basic settings in the ventilator’s breathing pattern are either volume-controlled ventilation (VCV 20-1500 ml/breath, typically 450-550 ml) or pressure-controlled ventilation (PCV 5-60 cm H2O in inspiratory pressure, typically 10-12 cm H2O). The different ventilator settings are described in detail below.
Examples of different ventilator settings are:
- Volume-Controlled Pressure Support (VCPS)
- Pressure Control (PCV)
- Volume Control (VCV)
- ASV – Adaptive Support Ventilation
- APRV
- Duo PAP breathing pattern
- Bi-vent/APRV
- SIMV (PC) + PS
- SIMV (VCPS) + PS
- Automode PC + PS
- Automode VCPS – VS
- Pressure Support (PS/CPAP)
- Volume Support (VS)
- Neurally Adjusted Ventilatory Assist (NAVA)
Ventilator treatment reduces the work of breathing but can unfortunately cause further damage to already diseased or injured lungs due to barotrauma, atelectasis, pneumothorax, or infections.
To minimize the risk of injury due to ventilator treatment, it is desirable to tailor the treatment for each individual patient and to minimize the load on lung tissue. Controlled ventilation involves significant changes in lung volume fluctuations and airway pressure. Gas exchange in the lungs is influenced by both ventilation and circulation. Common causes of respiratory failure include pulmonary edema, pneumonia, sepsis, atelectasis, severe COPD, or asthma. Other causes may include major trauma, lung contusions, head trauma, stroke, drug overdose, poisoning, unknown unconsciousness, or an exhausted patient. Controlled ventilation is typically performed with a ventilator. The patient is either endotracheally intubated (invasive ventilation) or ventilation is performed with a tightly sealed mask, non-invasive ventilation (NIV). The tube or breathing mask is connected to the ventilator’s tubing system and its respiratory gases.
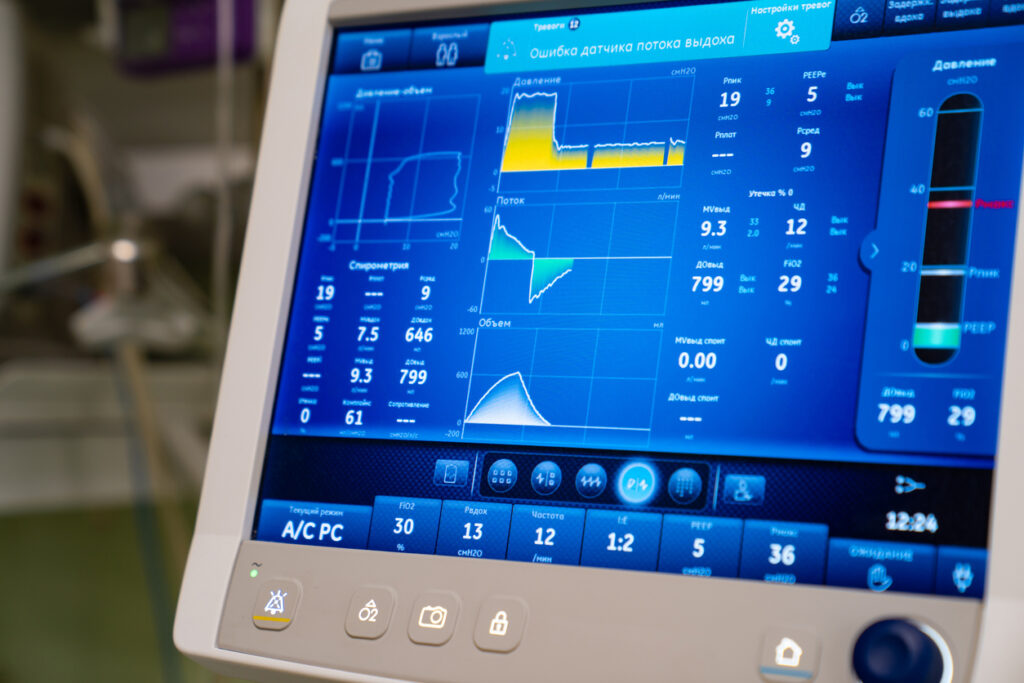
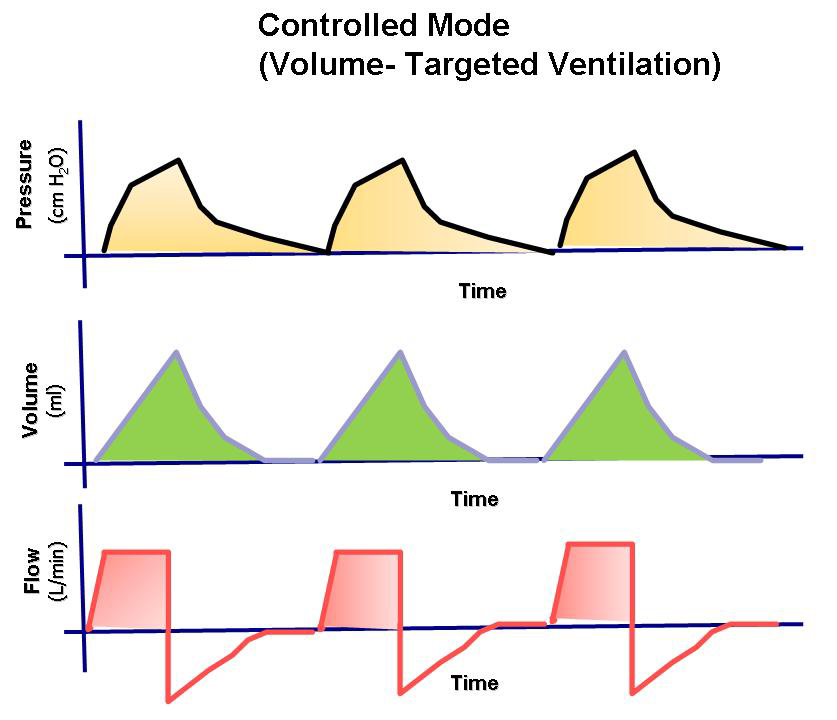
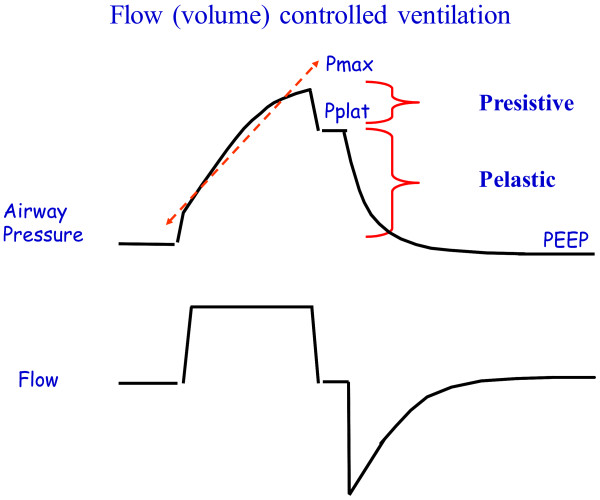
Ventilator treatment is usually volume-controlled (VC/VCV) or pressure-controlled (PC/PCV). Volume-controlled ventilation means that the given volume in each breath is predetermined, while pressure-controlled ventilation means that the given pressure in each breath is predetermined. Volume-controlled ventilation always delivers a constant flow of inhaled air, while in pressure-controlled ventilation, there is a decelerating flow. Pressure-controlled ventilation promotes gas distribution and results in lower peak pressure compared to volume-controlled ventilation. Volume-controlled ventilation reduces the risk of hypoventilation and can be beneficial in ARDS. The time a patient is treated with a ventilator is always minimized as much as possible.
Ventilator settings in different degrees of pulmonary injury
| Ventilator settings | Healthy lung | Moderate pulmonary failure | Severe pulmonary failure |
|---|---|---|---|
| Breathing mode | PS, VC, VCPC, BiPAP | PS, VC, PC, VCPC, BiPAP | PC, (PS, VCPC), BiPAP |
| Tidal volume ml/kg | < 6 - 8 | < 6 - 8 | < 6 - 8 |
| Respiratory rate | 15 - 20 | 15 - 20 | 15 - 30 |
| I:E quote | 1:2 | 1:2 - 1:1 | 1:1 - (2:1) |
| PEEP cm H20 | 0 - 5 | 5 - 10 | 10 - 20 |
| Oxygen fraction % | < 40 | 40 - 60 | 40 - 100 |
| Lung recruitement | - | Yes | Yes, at an early stage |
Ventilator Treatment in Severe Respiratory Failure
Patients Treated Outside ICU
- O2 supply with target SpO2 92-96%; for patients with COPD or risk of CO2 retention, target SpO2 88-92%1.
- When O2 supply, including supply with a reservoir mask, is insufficient, high flow via nasal cannula (HFNC, “Optiflow”) is recommended.
- When HFNC is insufficient, CPAP with pressure ≤ 10 cm H2O can be tried.
- Experience shows that COVID-19 patients who are entirely dependent on NIV, i.e., cannot maintain adequate gas exchange without NIV, often continue to deteriorate. This can lead to a need for urgent intubation, which increases patient risks and infection transmission risks. For patients with less pronounced lung failure, NIV can be an alternative used for longer periods, possibly alternating with HFNC. Similarly, NIV can be used for patients where intensive care is not indicated but should be avoided if palliative care is in place.
- HFNC, CPAP, and NIV carry a risk of aerosol formation and transmission, reinforcing the need for protective equipment. Compared to CPAP/NIV, HFNC may offer an advantage by requiring less close contact with the patient.
- HFNC and NIV should not be used during hospital transport; a reservoir mask should be used instead.
- Mobilization, cough assistance, and repositioning are important to prevent and treat impaired lung function. It has proven particularly effective with prone positioning, with or without other breathing support such as HFNC, NIV, or CPAP.
Potential Indications for Ventilator Care
- PaO2/FIO2 < 20 kPa or worsening with the need for increasing FIO2 (O2%), SpO2 <93% with O2 ≥ 10 L/min on a mask.
- Rising PCO2 (> 6.0 kPa), especially if pH < 7.30.
- Increased work of breathing and/or respiratory rate (RR) > 30/min. Ask the patient if breathing has improved or worsened over time.
- When NIV is used because oxygen on a reservoir mask or HFNC is insufficient treatment, and the patient continues to deteriorate or has not improved within 1-2 hours after treatment initiation.
- Impaired consciousness.
- Hypotension, oliguria, elevated and rising P-Lactate, echocardiogram with significant right and/or left heart failure.
- Before contacting the ICU, treatment limitations should normally have been discussed at the treating unit before the patient is placed on a ventilator. If the patient meets any ICU indication, ICU contact should be made in parallel with this discussion. Responsibility for this primarily lies with the responsible physician in the ward where the patient is being treated.
Intubation in Covid patients
The intubation procedure carries an increased risk of circulatory/respiratory collapse and infection of personnel, especially the one performing intubation. COVID-19 patients may appear relatively unaffected despite significant hypoxia and high respiratory rates. They can deteriorate very quickly and then have great difficulty recovering after intubation. Therefore, it is strongly recommended not to wait too long with intubation. Intubation of COVID-19 patients always carries an increased risk of infection spread. For procedure and checklist, see specific guidelines, https://sfai.se/download-attachment/11747.
Ventilation Care Recommendations
Humidification/Filter
Secret stagnation and “tube block” are relatively common; active humidification with conventional equipment is the preferred choice. If active humidification is not used, passive humidification with HME with a filter function is used. Always use a filter at the ventilator’s expiration port. All filter/hose changes are made with the ventilator on standby. Auto-PEEP and patient-ventilator dyssynchrony can be caused by filters that need changing, especially if the patient has active humidification and/or inhalations. Testing of new hoses can be skipped in consultation with the responsible physician.
Suction System
Closed system is always used in COVID-19 infection.
Tidal Volume/Driving Pressure
Generally, tidal volumes up to 8 ml/kg PBW are accepted if the driving pressure is ≤ 15 cm H2O and the plateau pressure is ≤ 30 cm H2O. Larger tidal volumes/driving pressures are accepted when reduction is impossible or requires measures that are deemed to worsen the situation, such as increased sedation, need for relaxation, or impaired gas exchange.
PEEP
Chosen individually, if compliance is good, often 6-12 cm H2O even with higher FIO2. Try higher PEEP if lower compliance and low PaO2/FIO2. Reassess high PEEP by reducing by 2 cm H2O and following up on tidal volume, compliance, and gas exchange. Note that compliance can only be assessed during controlled ventilation.
SpO2/PaO2 – Target Values:
- 88-94%, 7.5-9.5 kPa with controlled ventilation
- 92-94%, 8.5-9.5 kPa with assisted ventilation
PaCO2
Generally, up to 8.0 kPa is accepted. Higher PaCO2 can be accepted if pH > 7.20 with a reasonable breathing drive. Higher PaCO2 can also be accepted if the ventilation otherwise requires too high tidal volumes/driving pressure or significant auto-PEEP.
Lung Recruitment
Considered early if low PaO2/FIO2 and low compliance, especially if sudden deterioration occurs. Exclude bronchial intubation, secretion/threatening tube block, pneumothorax. Do not repeat recruitment if the previous attempt was ineffective.
Patient-Ventilator Dyssynchrony
Primarily managed by adjusting ventilator settings and increasing sedation, secondarily by intermittent or infusion muscle relaxants, which should be re-evaluated after 12-24 hours.
Prone Positioning
Recommended if PaO2/FIO2 < 20 kPa and also with PCO2 problems, aiming for at least 16 hours/day, with daily turning and 4-6 hours in the supine position.
Weaning
Due to slow improvement and risk of “setbacks,” formal weaning starts relatively late in the course and first with lower ventilator settings than usually the case.
Humidification/Use of Filters in the Ventilator Circuit (Tubes)
Balance between the risk of transmission and what is optimal for the patient.
- Make a patient- and situation-based choice between active and passive humidification. Active humidification is preferred. Indication for active humidification especially exists with pronounced hypercapnia requiring dead space elimination or with thick/dry secretions. Dry/thick secretions can cause tube block or auto-PEEP. If active humidification is not possible, consider acetylcysteine inhalations via a “closed” nebulization system.
- Passive humidification is done primarily with HME (heat-moisture exchanger), which also has a filter function. It should be placed as close to the tube as possible, but the closed suction system must be between the tube and the HME/filter.
- If an HME with a filter function is not available, another HME is used and complemented with a filter at the ventilator’s inspiratory outlet.
- There should always be a filter on the ventilator’s expiratory inlet.
- If active humidification is used, the HME/filter at the tube should not be used; instead, a filter should be placed at the ventilator’s inspiratory outlet.
- During all filter changes, hose changes, and similar, the tube should be briefly clamped, and the ventilator should be put on standby before disconnections are made. The ventilator is not restarted until it has been ensured that everything is reconnected. For tracheotomized patients, this is done similarly but without clamping.
Invasive Ventilation
- With pressure control, the driving pressure for the desired tidal volume is selected, then the respiratory rate (RR) is adjusted to achieve the desired minute ventilation and acceptable PaCO2, while avoiding auto-PEEP.
- Accept a tidal volume of approximately 8 ml/kg PBW (predicted body weight) if the driving pressure is ≤15 cm H2O (driving pressure = pressure above PEEP, the ventilating pressure). Gradually aim for a lower tidal volume/kg PBW if the driving pressure is higher.
- Note that driving pressure can only be properly assessed during controlled ventilation. In pressure-supported ventilation, it is suggested to accept tidal volumes up to 8 ml/kg PBW, provided that the support is max 14 cm H2O and the patient is not significantly “pulling” during inhalation. If this cannot be achieved, controlled ventilation or measures to reduce respiratory drive, such as increased sedation, are suggested.
- Note that reducing pressure support to lower tidal volume often has little effect but leads to increased work of breathing. Therefore, it is rarely appropriate to have pressure support < 8-10 cm H2O. For high tidal volumes, it may be appropriate to increase pressure support if the patient is working hard to breathe. The result is often unchanged tidal volume but with reduced work of breathing. Alternatively, switch to controlled ventilation.
- Higher tidal volumes/driving pressure are accepted when reduction is impossible or when alternatives, such as deeper sedation, muscle relaxation, worsened gas exchange, or pronounced patient-ventilator dyssynchrony, are considered to worsen the situation.
- The goal is peak pressure ≤ 30 cm H2O and driving pressure ≤ 15 cm H2O. Always aim for the lowest possible driving pressure.
- FiO2 with a target SpO2 of 88-94%, and 92-94% if pressure-supported ventilation is used.
- PEEP is selected individually, often 6-12 cm H2O. Lower PEEP is often chosen than in other ARDS cases, especially if the patient has high compliance (> 30 ml/cm H2O).
- If high FiO2 is needed, i.e., moderate to severe ARDS, higher PEEP may be tried, especially with low compliance. If higher PEEP does not improve gas exchange or compliance, or if increased PEEP leads to hemodynamic deterioration, revert to lower PEEP. Similarly, PEEP >8-10 cm H2O should be reconsidered daily, but first assess the effect of previous reduction attempts. Changes should be made in 2 cm H2O increments.
- Hypercapnia due to impaired CO2 elimination is accentuated by high PEEP, especially with relative hypovolemia. Consider giving volume and reducing PEEP, as a lower PEEP may overall be better even if it requires increasing FiO2.
- Consider early lung recruitment with increased PEEP and airway pressures if the patient has a low PaO2/FiO2 ratio and low compliance (< approx. 20 ml/cm H2O), but recruit cautiously in hypovolemia/hemodynamic instability. Do not repeat recruitment attempts if previous attempts were ineffective.
- Patient-Ventilator Dyssynchrony: When the patient is difficult to ventilate and does not follow the ventilator (“fights it”), it is managed with increased sedation (including increased opioid doses). If this is not enough, repeated doses of muscle relaxants or infusions for up to 24-48 hours can be tried. In cases of very severe gas exchange disorders, caution is advised when switching from supported to controlled ventilation. The risk is that this shift may cause respiratory collapse. The solution may be a quick return to spontaneous breathing with supported ventilation, e.g., with the reversal of medications.
Avoid aerosol generation by minimizing disconnection of ventilator tubes. This recommendation also aims to avoid derecruitment (atelectasis formation).
- Use a closed suction system.
- Avoid inhalation therapy unless strongly indicated.
- Minimize the number of bronchoscopies. Bronchoscopy is done for diagnosis and when there is an imminent risk of tube blockage. Use muscle relaxants during bronchoscopy, but be prepared for reversal (see the comment in the section on patient-ventilator dyssynchrony above). A blind protected brush is an alternative for diagnosis.
- If disconnection is unavoidable, set the ventilator to Stand-By and clamp the tube with forceps. Consider giving a sedation bolus before doing this. Switch to active ventilation only when all tubes are connected.
Prone positioning for at least 16 hours/day is recommended if PaO2/FiO2 < 20 kPa in the supine position. Prone positioning in COVID-19 ARDS often has a beneficial effect and can therefore be tried at higher PaO2/FiO2, for example, with gradual deterioration of oxygenation or when hypercapnia is more of a problem than hypoxemia. If true prone positioning is difficult to achieve, the prone side position is an alternative. In both cases, small adjustments should be made so that pressure points and the position of the head/neck change regularly.
Other ventilation modes: There are no studies showing clear benefits of using ventilation modes other than pressure control and pressure support. In the current situation, with varying levels of experience and competence among both doctors and nursing staff, it is recommended to refrain from using ventilation modes that are not commonly used. That is, use only pressure control and pressure support. Pressure-controlled ventilation with controlled tidal volumes, such as VCPS, is used in special cases where stable PaCO2 levels are required.
Sedation/Weaning
If the patient has well-functioning controlled ventilation, do not rush to switch to pressure support. Wait until PaO2/FiO2 ≥ 33 kPa (which corresponds to approximately SpO2 95% at FiO2 0.3) and ensure that the patient does not breathe with excessively large tidal volumes (e.g., > 10 ml/kg PBW) during supported ventilation. For the same reason, PEEP should not be reduced to <6 cm H2O until a relatively late stage in the process. A marked deterioration in oxygenation during turns indicates that the patient is not ready for extubation. Experience so far shows that patients with COVID-19 ARDS require at least 10-14 days of intensive care. During ongoing intensive care, the patient should be considered infectious. Extubation should be performed later in the process, i.e., when the need for continued respiratory support after extubation is assessed to be low.
Extubation
Several centers have reported airway obstructions after extubation, and it is unclear if and why this might be more common in COVID-19 patients than in other pneumonia/ARDS cases. Problems with secretion buildup are common after extubation and are managed typically with cough assistance and mobilization. Tracheotomy in selected cases could likely reduce the risk of reintubation and accelerate the end of intensive care. However, this assumes that the patient can be transferred to wards with the appropriate staffing and competence.
Refractory Hypoxemia/Hypercapnia
Possible interventions include recruitment maneuvers, prone positioning, optimizing PEEP (which may mean lowering PEEP), minimizing apparatus dead space, hemodynamic assessment/optimization (rule out hypovolemia as a cause of impaired CO2 elimination), deep sedation, neuromuscular blockade, treating fever, accepting spontaneous breathing/assisted ventilation despite larger tidal volumes/airway pressures than desired, inhalation of vasodilatory medications (there are positive experiences with inhalation of iloprost and milrinone), and consultation with ECMO specialists. A high incidence of pulmonary embolism has been described in COVID-19 patients, strengthening the indication for diagnostics concerning this.
ECMO
Consider contacting ECMO if the patient does not improve with the previously mentioned interventions and severe hypoxemia persists (e.g., PaO2/FiO2 <10 KPa) and no contraindications are present. The indication for ECMO may change during the epidemic.
Tracheotomy
Use protective equipment, administer muscle relaxants to avoid coughing, preferably cover the face/tube with a plastic drape, and set the ventilator to stand-by when the tube is retracted, and the trachea is incised. Ensure that all tubes are connected, and the cannula is cuffed before restarting the ventilator. See the link for a description of ARDS in COVID-19 and references.
Ventilator Treatment Graphics
Click the image to download the PDF file

Ventilator treatment in ARDS
Definition of ARDS
- Acute lung failure (≤ 7 days)
- The lung failure is not fully explained by heart failure
- Bilateral infiltrates (X-ray/CT/ultrasound)
- PaO2/FiO2 < 40 kPa despite PEEP 5 cm H2O
Grading of ARDS
| ARDS | PFI (kPa) = PaO2/FiO2 | FiO2 with PaO2 10 (SpO2 ≈ 95 %) | FiO2 with PaO2 8 (SpO2 ≈ 90 %) |
|---|---|---|---|
| Mild | 40.0-26.6 | 25 % | 21 % |
| Moderate | 26.6-13.3 | 37 % | 30 % |
| Severe | ≤ 13.3 | 75 % | 60 % |
Overarching Recommendations
- Avoid positive fluid balance if possible, increasingly important with higher degrees of ARDS.
- With increasing ARDS severity, there is a growing need for hemodynamic evaluation (heart echo) and special measures if right heart failure occurs.
- Ensure adequate diagnosis and treatment of the etiology, infection diagnostics, and infection treatment.
- Choose the level of sedation for patient comfort, avoid excessive respiratory effort (if using PS), and prevent patient-ventilator dyssynchrony.
- Prophylaxis for DVT, stress ulcers, VAP, and pressure ulcers
Decision support at early ARDS on ventilator treatment
| Classification | Actions | Treatment goals |
|---|---|---|
| Mild ARDS PFI 26.7-40 kPa | Favorable body position If not intubated: High flow halter ≥ 40 L/min alt. NIV with PEEP ≥ 6, TU ≤ 5. If intubated: Often TU/CPAP: PEEP ≥ 8, TV ≤ 8 ml/kg Initiate adequate antimicrobial therapy Avoid positive fluid balance if possible | Target: SpO 2 : 92-95%, PaO 2 : 9-10 RR < 7 Treatment aims for • Reduce heavy breathing work • Avoid large tidal volumes • Minimize patient/ventilator dyssynchronies If not better in NIV ≤ 2 h ➔ possible intubation |
| Moderate ARDS PFI 26.7-13.3 kPa | Frequent need for intubation, especially if PFI < 20: • Usually PEEP 10-14 • Dimensions TV: ≤ 6 ml/kg PBW • Most often controlled ventilation • Ventilating pressure: if PS: 8-14, if PC: ≤ 15 • If heavy breathing drive / large TV, increase sedation In PFI <20: abdominal position 16-20 h/day (unless contraindicated) | Target: SpO 2 90-94%, PaO2 8-10, At PC: pH > 7.25 PaCO2: ≤ 7(-8) In case of negative PFI trend despite adequate treatment, consider and manage as severe ARDS. Aim also as in the action box on the left. |
| Severe ARDS PFI <13.3 kPa | Invasive ventilation in controlled mode (PC) TV ≤ 6 ml/kg PBW TK over PEEP: ≤ 15 Ptop <30, Usually PEEP 12-18, titrated (Note compliance and hemodynamics) RR determines MV (target pH > 7.25, PCO2 <7-8) Unless contraindicated: • Abdominal position 16-20 h/day Sedation to comfort and knocked out self-breathing • Neuromuscular blockade of severe dysynchrony Cardiac echo for hemodynamic assessment • ECMO contact in case of continued negative trend (PFI <10) | Target: SpO 2 88-94%, PaO2 7.5-10 pH > 7.25 PaCO2: ≤ 7-8 • Avoid self-breathing • If possible, negative fluid balance • Caution when recruiting with a ventilator, but try in case of sudden deterioration and as a rescue measure • Hemodynamic optimization, right ventricular relief |
ARDS
In ARDS, there is often, but not always, a correlation between the degree of oxygenation problems (lower PaO2/FiO2) and lower compliance. This is due to large lung areas that are not gas-filled/ventilated. When a smaller portion of the lungs is ventilated, compliance is low, and blood flow through unventilated areas becomes an intrapulmonary shunt, which in turn explains hypoxemia that responds poorly to increased FiO2. With this pathophysiology, improved oxygenation and compliance are often, but not always, seen with increased PEEP and after lung recruitment with high airway pressures. The mechanism is that previously non-gas-filled/ventilated lung areas open up, which means larger parts of the lung are ventilated, resulting in better compliance and less shunting. This is the logic behind linking the need for increased FiO2 to the use of higher PEEP (PEEP-FiO2 tables). Over recent months, there has been an intense discussion about whether COVID-19-induced ARDS systematically differs from ARDS caused by other pneumonia, for example. In several relatively large patient materials, it has been difficult to demonstrate clinically significant differences, such as in compliance.
At the same time, we have also locally experienced that it is relatively common to see a combination of severe ARDS (low PaO2/FiO2 ratio) and relatively high (almost normal) compliance, though this can also occur in ARDS of other etiologies than COVID-19. For all patients with ARDS, regardless of etiology, treatment choices, such as ventilator settings, must be continuously adapted to the current situation. One example is when severe oxygenation problems (low PaO2/FiO2) are associated with well-preserved, almost normal compliance. Preserved compliance indicates that low PaO2/FiO2 is not explained by unventilated lung areas, which in turn means that there is less potential for higher PEEP to improve gas exchange or compliance. Preserved compliance and the absence of large, non-gas-filled/ventilated lung areas are consistent with the most common radiographic findings in COVID-19 pneumonia: ground-glass infiltrates and the absence of larger consolidated lung areas.
In this situation, the oxygenation problem has instead been suggested to be due to V/Q mismatch combined with inhibited hypoxic vasoconstriction. It is in this situation that slightly lower PEEP and slightly higher tidal volumes are reasonable. High PEEP can impair gas exchange, especially CO2 elimination, through several mechanisms. This is not specific to COVID-19 patients, but it becomesespecially important since PEEP seems less likely to open unventilated lung areas. Impaired gas exchange with increased PEEP is more pronounced if the patient is relatively hypovolemic. With preserved lung compliance, PEEP has a greater effect on preload than in ARDS with reduced compliance. Reduced cardiac output due to high PEEP and relative hypovolemia has been suggested to contribute to acute kidney failure in COVID-19 patients. In other COVID-19 pneumonia patients, a pathophysiology more typical of “usual” ARDS is described: reduced compliance and shunt due to non-gas-filled/ventilated lung areas combined with improved gas exchange with increased PEEP/lung recruitment. In this situation, the indication for limiting tidal volume and trying higher PEEP is strengthened.
The discussion about different types of ARDS will likely continue. Regardless, the overall experience seems to suggest greater restraint with higher PEEP and acceptance of higher tidal volumes in COVID-19 ARDS, but also that there may be patients/situations where the pathophysiology more closely resembles “usual” ARDS.
Literature
| References respiratory failure Covid-19 Corona |
|---|
| O’Driscoll BR, Howard LS, Earis J, Mak V, British Thoracic Society Emergency Oxygen Guideline G, Group BTSEOGD. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1-ii90 |
| Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. |
| Peng PWH, Ho PL, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anaesth. 2020;124(5):497-501. |
| Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4). |
| Murthy S, Gomersall CD, Fowler RA. Care for Critically Ill Patients With COVID-19. JAMA. 2020;323(15):1499-500. |
| Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, et al. Prevention of endotracheal suctioninginduced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med. 2003;167(9):1215-24. |
| Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. |
| Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Eng J Med. 2015;372(8):747-55. |
| Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine |
| Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017;195(9):1253-63. |
| Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatment for different phenotypes? . Intensive Care Med. 2020. |
| Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D. Covid-19 Does Not Lead to a ”Typical” Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020. |
| Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am J Respir Crit Care Med. 2020;201(12):1560-4. |
| Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. |
| Intensive Care Med. 2020. Tobin MJ. Basing Respiratory Management of COVID-19 on Physiological Principles. Am J Respir Crit Care Med. 2020;201(11):1319-20. |
| Grieco DL, Bongiovanni F, Chen L, Menga LS, Cutuli SL, Pintaudi G, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care. 2020;24(1):529. |
| Raoof S, Nava S, Carpati C, Hill NS. High-Flow, Noninvasive Ventilation and Awake (Nonintubation) Proning in Patients With Coronavirus Disease 2019 With Respiratory Failure. Chest. 2020. |
| Camporota L, Sanderson B, Dixon A, Vasques F, Jones A, Shankar-Hari M. Outcomes in mechanically ventilated patients with hypoxaemic respiratory failure caused by COVID-19. Br J Anaesth. 2020. |
| Marini JJ, Dellinger RP, Brodie D. Integrating the evidence: confronting the COVID-19 elephant. Intensive Care Med. 2020;46(10):1904-7. |
| Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816-21. |
| Suffredini DA, Allison MG. A Rationale for Use of High Flow Nasal Cannula for Select Patients With Suspected or Confirmed Severe Acute Respiratory Syndrome Coronavirus-2 Infection. J Intensive Care Med. 2020:885066620956630. |
| Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020. |
| Coppo A, Bellani G, Winterton D, Di Pierro M, Soria A, Faverio P, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-74. |
| Tobin MJ, Laghi F, Jubran A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am J Respir Crit Care Med. 2020;202(3):356-60. |
| Larsson E, Brattstrom O, Agvald-Ohman C, Grip J, Campoccia Jalde F, Stralin K, et al. Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol Scand. 2020. |
| Paul V, Patel S, Royse M, Odish M, Malhotra A, Koenig S. Proning in Non-Intubated (PINI) in Times of COVID-19: Case Series and a Review. J Intensive Care Med. 2020;35(8):818-24. |
Fine Tuning in the Ventilator
It is essential to remember that normal human breathing is a physiological masterpiece. A large diffusion surface in the lungs within a relatively small cavity cooperates with both an efficient muscle motor and the blood flow in the pulmonary circulation. This combination primarily regulates oxygenation and CO2 elimination. Breathing is centrally controlled by efferent and afferent neurophysiological pathways that can react and adjust breathing instantly as needed. Ventilation and perfusion form a finely tuned system that has evolved over millions of years. Ventilation in the ventilator, however, is entirely dependent on our settings in a mechanical ventilator under positive pressure ventilation. Modern ventilator treatment is, at best, a crude imitation of natural breathing, although it is the foundation of modern intensive care.
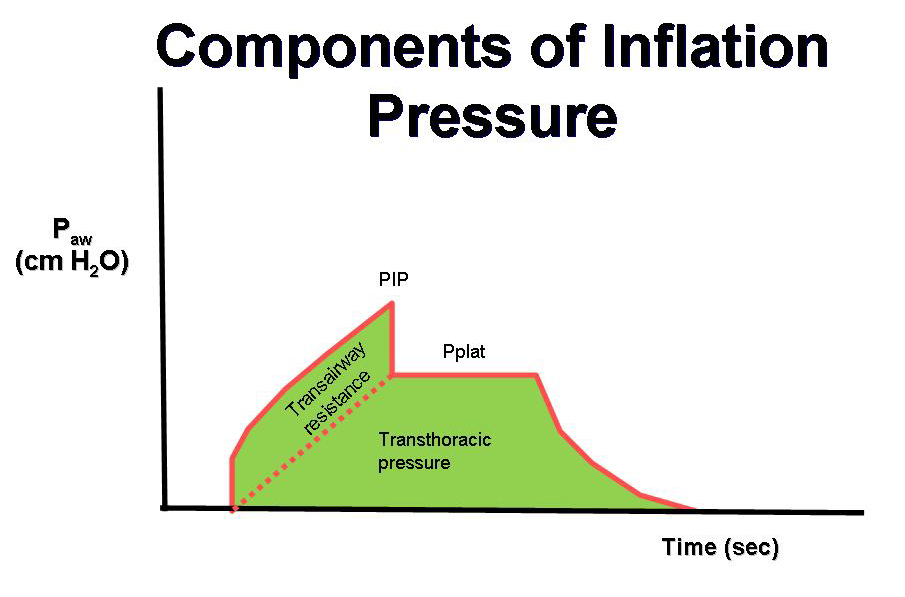
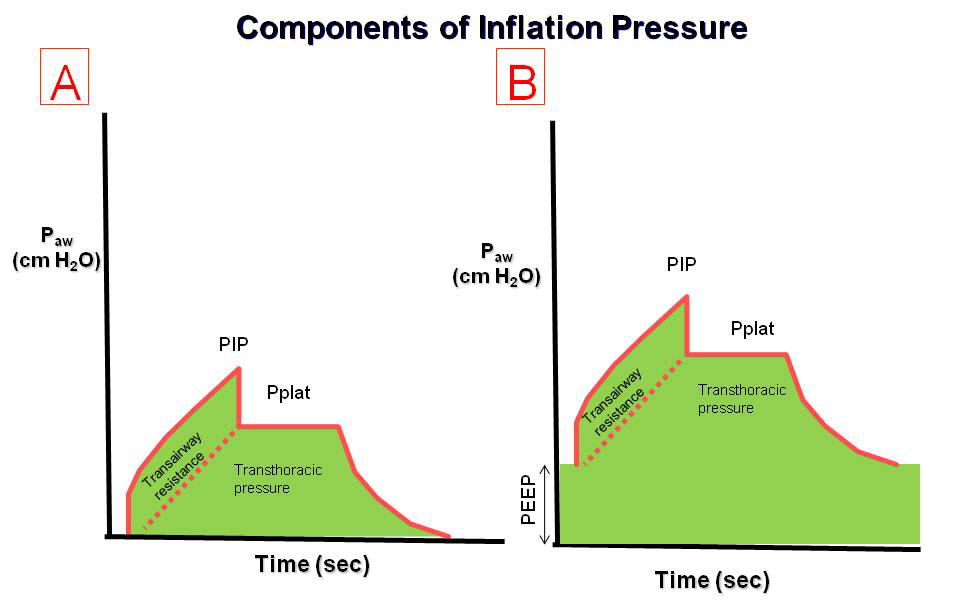
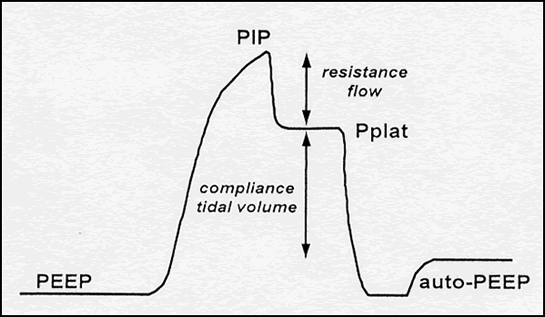
In the simplest case of volume-controlled ventilation (VC), where the patient does not contribute with their own breathing, the ventilator ensures oxygenation and CO2 elimination with constant volume but varying insufflation pressure. Ventilator treatment involves positive pressure ventilation of the lungs, while normal breathing is a form of negative pressure ventilation. It is the positive pressure that, in various ways, damages the lungs during prolonged ventilator treatment. The goal of modern ventilator treatment is, therefore, to limit ventilator-induced lung injury (VILI) and prevent the patient from injuring themselves through prolonged increased breathing effort (“patient self-inflicted lung injury, P-SILI”). Limiting lung injury is only possible as long as we stay within the safety margins regarding tidal volume, peak pressure, and transpulmonary driving pressure.
During assisted ventilation (ASB/PS/CPAP), the patient controls their own breathing, which requires increased attention and adaptation of ventilator settings to an individual and dynamic need. The most commonly used form of assisted ventilation is probably pressure-supported ventilation, also called PS/CPAP. Other assisted ventilation modes, such as NAVA, APRV, and PAV, are available but are used less frequently. However, most ICU patients with more than a day’s ventilator time will spend part of their weaning phase in PS/CPAP.
Adjustable Parameters in PS/CPAP
- Trigger sensitivity
- PEEP
- Pressure support (= pressure above PEEP)
- Inspiratory rise time
- Inspiratory cycling off
Pressure control (PC) breaths always function as a backup mode under PS/CPAP. This breathing function takes over with controlled breaths when no breathing attempts are made during a preset apnea time.
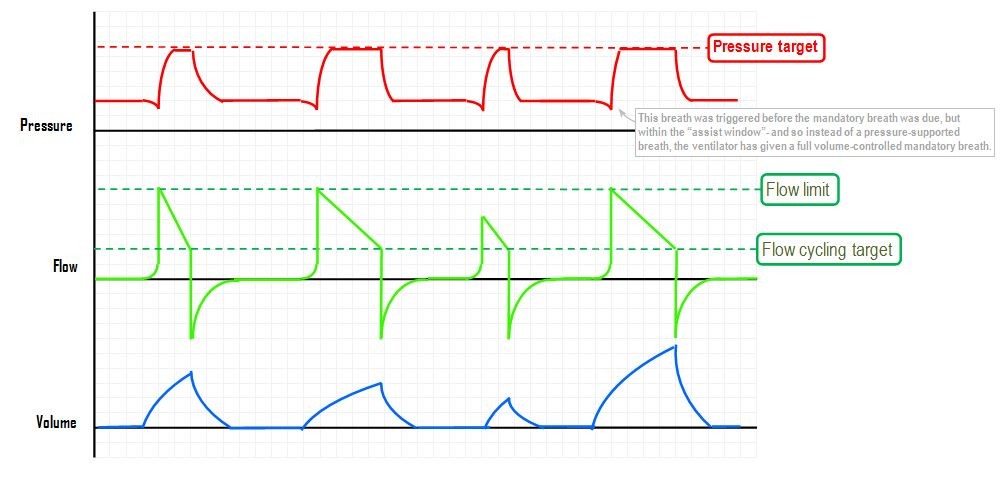
Pressure Supported Ventilation (PS)
The inspiratory rise time defines the time from the trigger of the breath until the maximum inspiratory flow is reached. Inspiratory cycling off describes the point where inhalation transitions to exhalation as a percentage of maximum inspiratory flow.
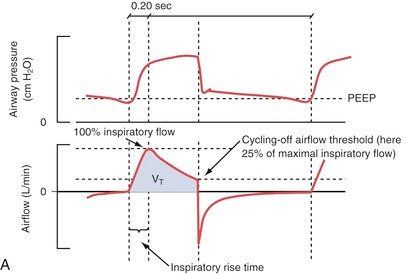
Trigger Sensitivity
Trigger sensitivity determines how a breath is initiated: In PS/CPAP, the ventilator uses a pneumatic trigger that is either flow- or pressure-based. During flow-based triggering, the ventilator detects an inspiratory effort through a measured reversal in expiratory flow. To register and measure the reversal, the ventilator uses a bias flow. This means that the ventilator supplies an airflow of 2 L/min during exhalation that runs parallel to the expiratory flow. This provides a constant “baseline” that is measurable and not individual or variable like a physiological exhalation. When the patient initiates a breath, this bias flow decreases relative to the patient’s inspiration. The change in airflow triggers an assisted breath when it exceeds the preset trigger value. A reasonable default setting in PS/CPAP could be 1.4 L/min. A lower value increases sensitivity and can improve patient comfort by making it easier for the patient to trigger each breath. However, there is a risk of auto-triggering with very low trigger sensitivity due to movements or leaks in the breathing circuit, which increases the lower the threshold is set. This can create a dyssynchronous breathing pattern that not only stresses the patient but also increases the risk of ventilator-induced lung injury.
In pressure-based triggering, a breath is initiated when the patient creates a negative pressure below the preset PEEP value through an inspiratory effort. The principle is “simple” and works in most ventilators. The downside is that it often increases the ventilator’s response time and can be burdensome for the patient with increased work of breathing compared to flow-based triggering. The risk of self-triggered breaths seems to be lower in pressure-based trigger settings, even with a low threshold. However, a high trigger value can challenge the patient or even prevent breaths from being triggered at all. A trigger set too high makes spontaneous breathing more difficult and can be unpleasant for the patient.
There are very few scientific studies on different trigger settings evaluated against clinical outcome measures. Flow-based triggering appears to be a more modern and smarter variant. The recommendation could thus be to avoid changing the preset flow trigger unless there is a clinically obvious triggering problem.
PEEP
The basic idea of setting PEEP is simple: always maintain an intra-alveolar pressure above zero cm H2O to prevent end-expiratory collapse of lung tissue. The goal, hypothetically, is a global (throughout the lung), constant (throughout the breathing cycle), and persistent (throughout the treatment period) ventilation/perfusion ratio (V/Q) that is as close to 1 as possible. The task is to find the right PEEP level that is neither too low nor too high, an optimal PEEP.
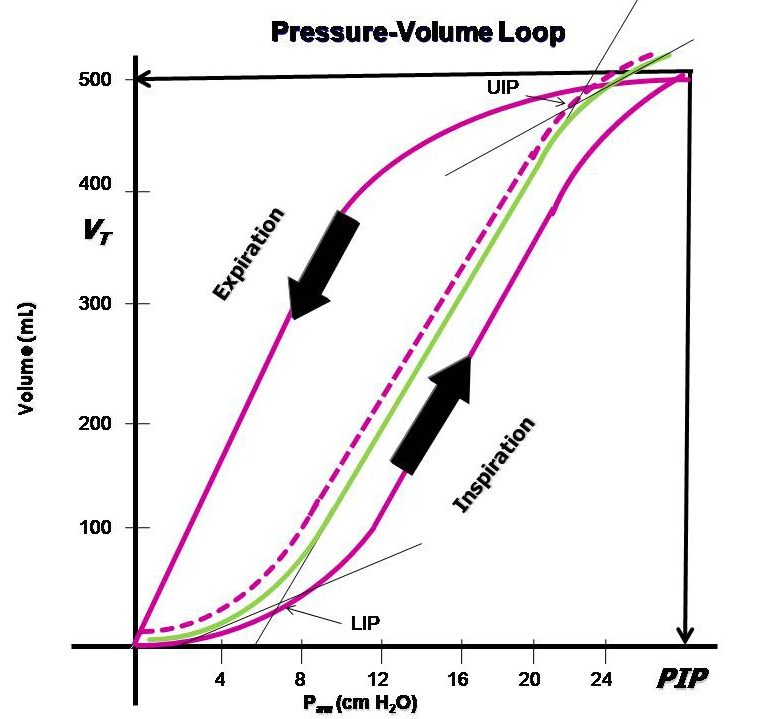
Too low PEEP can increase the risk of shunt formation, dynamic “recruitment/derecruitment,” leading to “shearing stress” and atelectatic trauma, which in turn increases the risk of lung injury and ARDS development. Too high PEEP can increase the risk of end-tidal lung overdistension with increased dead space, which may then create a need for increased driving pressure (> 15-20 cm H2O). The best method for finding the right PEEP level during controlled ventilation is debated, and no conclusive data is yet available. Several methods are also relatively complicated in practice.
A fundamental problem with all PEEP methodologies is that an immediate improvement in lung mechanics or gas exchange does not necessarily imply an improvement over time. Regarding ventilator-induced lung injury and ARDS, the benefit or harm of a given change may sometimes be assessed only after several days of treatment. It should also be remembered that all pressures measured during ventilator treatment are global measures. The patient’s lungs are subjected to different pressures in various parts while elasticity, compliance, and atelectasis susceptibility also vary in different areas of the lung.
What is added during assisted ventilation is that the actual pressure the lung is exposed to during inspiration, the transpulmonary pressure, cannot yet be measured easily, though method development is underway. Moreover, assisted ventilation is patient-controlled and, thus, variable in both volume and dynamics. This makes most bedside measurement methods used in the past irrelevant or impossible to interpret regarding PEEP and PS/CPAP.
- Static compliance
- Lowest inflection point
- Lowest shunt
- Lowest dead space fraction
Although esophageal manometry with a balloon seems like a good method for measuring transpulmonary pressure with significant clinical potential for guiding ventilator treatment, it does not seem to have any routine use for finding the right PEEP level during assisted ventilation.
Electrical impedance tomography (EIT) appears to be a more practically promising and usable method for PEEP adjustment, both in controlled and assisted ventilation. However, despite the technology not being new and its successful use in several clinical studies, it is still far from routine use.
In summary, regarding PEEP, there is not much concrete science to help us find the right level. As a guide, one can study trends on the ventilator, looking for changes in dynamic compliance over the last 24 hours. For example, recurring deteriorations that can be related to turning the patient, who is otherwise pain-free and unstressed, may indicate an increased atelectasis tendency. This patient would most likely benefit from increased PEEP. In cases of low dynamic compliance requiring high driving pressure to achieve adequate tidal volumes, and a patient who needs to use accessory respiratory muscles, a reduction in PEEP might lead to immediate improvement. Regardless of which way we adjust PEEP, we should follow and evaluate changes in dynamic respiratory measurements over time in conjunction with the clinical picture.
Pressure Supported Ventilation (PSV)
The inspiratory rise time defines the period from when a breath is triggered until maximum inspiratory flow is reached. The inspiratory cycling-off threshold describes the point where inhalation transitions to exhalation as a percentage of the maximum inspiratory flow.

Trigger Sensitivity
How a breath is triggered: In PSV/CPAP, the ventilator uses a pneumatic trigger, which can be either flow-based or pressure-based. In flow-based triggering, the ventilator detects an inhalation attempt by measuring a reversal in expiratory flow. To register and measure this reversal, the ventilator uses a so-called bias flow. This means that the ventilator delivers a flow of 2 L/min during exhalation, running parallel to the expiratory flow. This provides a constant “baseline” that is measurable and independent, unlike physiological exhalation, which varies. When the patient initiates a breath, this bias flow decreases relative to the patient’s inspiration. The change in flow triggers an assisted breath when it passes the pre-set trigger value. A typical default setting in PSV/CPAP might be 1.4 L/min. A lower value increases sensitivity and response time, enhancing patient comfort by making it easier for the patient to trigger each breath. However, one should be aware of the risk of auto-triggering with very low trigger sensitivity, caused by movements or leaks in the breathing circuit, which increases as the threshold is lowered. This can create a dyssynchronous breathing pattern, which, besides stressing the patient, also increases the risk of ventilator-induced lung injury.
In pressure-based triggering, a breath is initiated when the patient creates a negative pressure below the pre-set PEEP value. The principle is “simple” and works in most ventilators. However, it often increases the ventilator’s response time and can be more burdensome for the patient due to the increased work of breathing, compared to flow-based triggering. The risk of self-triggered breaths seems lower with pressure-based triggering, even at a low threshold setting. However, setting the trigger too high can really challenge the patient or even prevent breath triggering entirely. A too-high trigger setting impairs spontaneous breathing and can be uncomfortable for the patient.
There are very few scientific studies evaluating different trigger settings in relation to clinical outcomes. Flow-based triggering appears to be a more modern and smarter variant. The recommendation would thus be not to change the pre-set flow trigger unless there is a clinically apparent triggering issue.
PEEP
The basic principle for PEEP settings is simple: to always maintain an intra-alveolar pressure above zero cm H2O to prevent end-expiratory collapse of lung tissue. The goal is, hypothetically, a global (across the whole lung), constant (throughout the respiratory cycle), and persistent (throughout the treatment period) ventilation/perfusion ratio (V/Q) as close to one as possible. The challenge is thus to find the right PEEP level that is neither too low nor too high—what is termed “optimal PEEP.”
A too-low PEEP can increase the risk of shunt development, dynamic “recruitment/derecruitment,” leading to “shearing stress” and atelectatic trauma, which in turn increases the risk of lung injury and ARDS development. A too-high PEEP can increase the risk of end-tidal overdistension of the lungs, creating more dead space, which in turn may create a need for increased driving pressure (> 15-20 cm H2O). The best method for finding the right PEEP level during controlled ventilation is debated, with no conclusive data yet. Several methods are also relatively complicated in practice.
A fundamental problem with all PEEP methodologies is that a momentary improvement in lung mechanics or gas exchange does not necessarily imply an improvement over time. Regarding ventilator-induced lung injury and ARDS, the benefits or harm of a given change may sometimes only be assessed after several days of treatment. It’s also important to remember that all pressures measured in connection with mechanical ventilation are global measures. However, the patient’s lung is subjected to varying pressures in different parts, with elasticity, compliance, and atelectasis tendency also varying across different lung regions.
With assisted ventilation, the pressure the lung is actually exposed to—transpulmonary pressure—cannot yet be easily measured, though method development is ongoing. Additionally, assisted ventilation is patient-controlled, leading to variability in both volume and dynamics. This makes it challenging, if not impossible, to draw conclusions from most bedside measurement methods used. Thus, when it comes to PEEP and PSV/CPAP, we have relatively little use for measuring:
- Static compliance
- Lowest inflection point
- Lowest shunt
- Lowest dead space fraction
Although esophageal balloon manometry appears to be a good method for measuring transpulmonary pressure with great clinical potential to guide ventilator management, it does not seem to have a routine place in finding the right PEEP level during assisted ventilation.
Electrical impedance tomography (EIT) seems to be a more practically promising and useful method for PEEP adjustment in both controlled and assisted ventilation. Despite the technique not being new and having been successfully used in several clinical studies, it is still far from routine use.
In summary, when it comes to PEEP, there is not much concrete science to help us find the right level. In clinical practice, studying the trend of dynamic compliance changes over the last 24 hours can be helpful. For example, recurring deteriorations related to patient repositioning in an otherwise pain-free and non-stressed patient may indicate increased atelectasis propensity. Such a patient would likely benefit from increased PEEP. If dynamic compliance is low, requiring high driving pressure to achieve adequate tidal volumes, and the patient needs to use accessory respiratory muscles, lowering PEEP may lead to immediate improvement. Regardless of whether we adjust PEEP up or down, we should monitor and evaluate changes in dynamic respiratory parameters over time, along with the clinical picture.
Pressure Support
When transitioning from pressure-controlled ventilation (or PCV) to pressure support ventilation, the level of support (PS above PEEP, “driving pressure”) can often be reduced. This is because the patient begins to contribute to their own breathing through diaphragmatic contractions. In pressure support ventilation, the pressure during the inspiratory phase remains constant while the flow decreases. As long as the PS is set at a level where the patient is not “pressure support dependent,” the patient can control their tidal volume (VT) themselves. However, if the level is reduced to the point where the patient can no longer compensate through their own effort, the VT will decrease in proportion to the pressure support.
The general goals for adjusting PS are to aim for a respiratory rate under 30/min and a tidal volume under 6 ml/kg (IBW). Furthermore, the goal is for breathing to occur without the use of accessory respiratory muscles and for the patient to be “calm” and unstressed. Assessing this last point is, of course, challenging, as there are often many factors beyond ventilator treatment that can stress the patient.
Weaning from the ventilator
During weaning, pressure support should be kept below 20 cm H2O. The support level where adequate tidal volumes are achieved is not necessarily the level the patient must maintain. The goal during weaning can be to maximize training effect with minimal effort. A protocol-driven weaning process with structured tapering of support and, ultimately, extubation has been shown in literature to result in more ventilator-free days. Since ventilator treatment and weaning are often doctor-directed based on individual decisions, standardized weaning protocols are lacking in most clinics across the country. A pragmatic approach is to regularly evaluate and possibly reduce PS until the patient needs to increase their work of breathing. Signs of this could include an increased respiratory rate or visible use of accessory respiratory muscles, often most clearly seen in the sternocleidomastoid muscle. Another good method is, of course, to talk with the patient, who is often communicable during the weaning phase, and ask them to signal when breathing starts to feel too difficult. The support level chosen should be just above the effort threshold. The patient should never “struggle” on the ventilator and risk becoming exhausted. Extubating an exhausted patient is contraindicated. Acceptance of 5 over 5 in support pressure/PEEP with FiO2 below 0.3 is considered a safe measure for extubation. Higher pressure levels may also be acceptable for extubation in some cases. It is always a clear advantage if the patient is communicable and calm during extubation. “Crash extubation” should be avoided.
Rise Time
The set pressure or airflow that assists the patient during inhalation (pressure above PEEP in PS/CPAP mode) is not maximal from the start but increases gradually after the breath has been triggered. The speed of this increase is set using the inspiratory rise time.
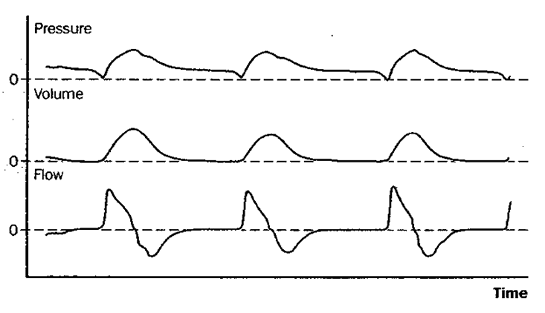
Pressure Supported Ventilation (PS)
Shortening the inspiratory rise time (A+B) leads to a decreased inspiration time without significant changes in tidal volume (VT) by shifting the inspiratory curve to the left. Increasing the inspiratory cutoff (C+D) reduces both the inhalation time and VT through earlier “cycling-off”.
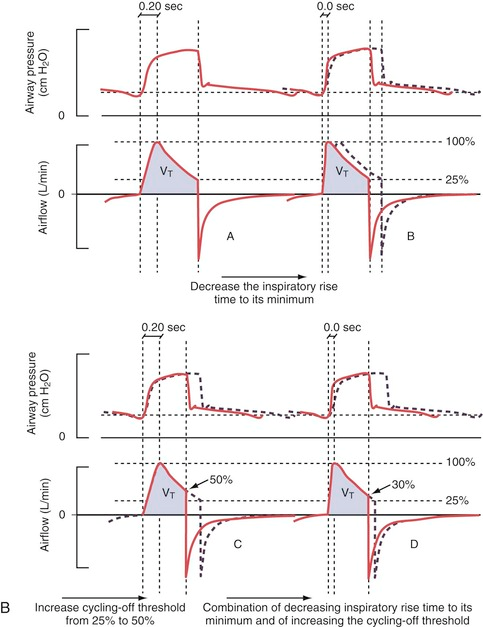
The default setting for rise time in PS/CPAP is 0.15 seconds. A decrease in the set inspiratory rise time theoretically does not affect the tidal volume. However, this is only “in theory” because an inadequate shortening of the rise time can cause the airflow to be interrupted prematurely, resulting in sporadic double breaths (“double triggering”). This occurs due to excessive resistance in the breathing circuit caused by secretions or the patient “resisting” (“flow in excess of demand,” see curve A below).
Double breaths do not necessarily indicate a need for increased inspiratory rise time and must always be related to the clinical situation. Prolonging the inspiratory rise time can be advantageous in patients with ARDS and a tendency toward atelectasis, as it provides time for adequate redistribution of tidal volume between alveoli with different time constants. However, too long a rise time can lead to a feeling of breathlessness and increased work of breathing. This can be visible as a concave deflection of the inspiratory pressure curve (curve C below).
Examples of dyssynchrony due to inadequate inspiratory rise time. (A) in the figure shows short inspiratory rise time (0.0 sec) with an early pressure peak during the inspiratory phase as a sign of “flow in excess of demand” or obstruction in the airways or tube. (B) shows a normal inspiratory curve. (C) shows a long inspiratory rise time (0.5 sec) with a concave deformation of the curve during the flow increase, indicating “demand in excess of flow” with consequently increased work of breathing.
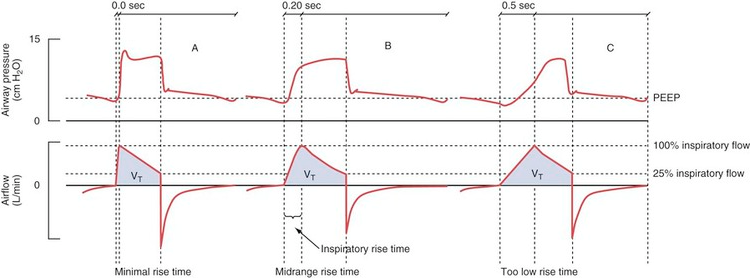
Generally speaking, a short inspiratory rise time (shortened inhalation relative to exhalation time) is especially beneficial for obstructive patients (asthma, COPD), while a prolonged rise time can sometimes be beneficial for patients with a tendency toward atelectasis and a slow breathing rate.
Inspiratory Cutoff
Airflow during inhalation begins to decrease as soon as the preset pressure level is reached. When the flow falls below a certain level, expressed as a percentage of the maximum flow, inspiration transitions to expiration (referred to as “cycling off”). The preset value is 30%. By increasing the value through changes to the inspiratory cutoff, the patient’s breath becomes shorter and vice versa.
Increased inspiratory cutoff to 50% (C) with consequent reduced tidal volume (VT). Image (D) shows an attempt to compensate VT by reducing the inspiratory cutoff to 30% and shortening the inspiratory rise time to 0.0 sec.
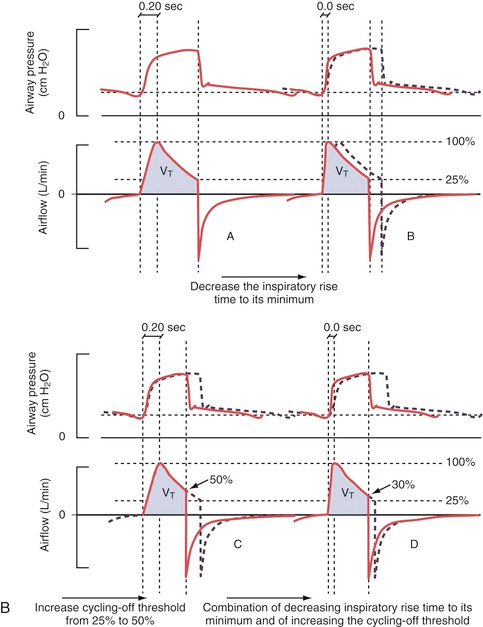
The goal is clearly to influence the I:E ratio. To give the obstructive patient more time to exhale and reduce the risk of auto-PEEP (increased inspiratory cutoff, preferably combined with a shortened inspiratory rise time) or to increase inspiratory time for the atelectasis-prone restrictive patient with low compliance (reduced inspiratory cutoff with a possible extension of the inspiratory rise time). Double breaths may indicate a need for further reduction of the inspiratory cutoff.
In Summary
Fine-tuning ventilator settings allows us to optimize ventilatory treatment for individual patients. Although there are alternatives to PS/CPAP, this mode of ventilation is generally considered the most effective weaning pattern. Default settings in PS/CPAP should form the basis of ventilator settings, but adjustments may be required based on specific lung pathologies and can vary during different phases of weaning. To achieve effective weaning from ventilator support, active presence with relevant adjustments to ventilator settings is necessary, along with some acceptance of the “trial and error” principle, while considering lung physiological principles and continuous monitoring of respiration and the patient’s overall clinical picture.
Several different measurement methods exist for finding optimal ventilator settings, but basic clinical examination, supplemented with ultrasound, frequent blood gas analyses, and standard X-rays, is usually sufficient to optimize ventilation. CT or MRI can, in some cases with restrictive elements, further improve the understanding of the patient’s lung disease and should be performed relatively liberally in intensive care patients.
Weaning from Ventilator – Practical Advice
Active weaning is recommended only during daytime between 07-22
Criteria for Starting Active Weaning
- Parameters within target orders and clinically stable patient
- TU/CPAP last 12 hours, FIO2 ≤ 50%, PEEP ≤ 15, TU ≤ 20, stable blood gases
- Stable circulation with reduced/unchanged inotropic support
- Decreasing infection parameters
- Temperature < 38.5°C
- Not too large cumulative positive fluid balance
- Digestive system functioning
- Pain controlled – low doses of opioids
- RASS -3 or higher
PEEP
- Reduce PEEP by 2 cm every two hours (maximum 6 times/day) until PEEP reaches 5-8 and adjust oxygen according to need
- If FIO2 > 40%, maintain PEEP ≥ 10
Pressure Support
- Reduce TU by 2 every four hours, as long as TV is over 6 ml/kg (maximum 4 times/day) until TU reaches 8
- Blood gas analysis before new adjustment and 30 minutes after
If the Patient Shows Signs of
- Stress/anxiety
- Pain
- Deviating values from target orders
- Increased respiratory rate > 30
Consider Actions
- For respiratory deterioration, revert to previous ventilator settings
- Consider causes other than respiratory
Criteria for Extubation
- Awake patient
- Can squeeze hands on command
- Can make eye contact
- Adequate cough reflex
- Not too much mucus
- If PEEP 8 and oxygen 35%, the patient can be extubated but will need NIV
- If PEEP 5 and oxygen 25%, the patient is expected to manage breathing with 1-2 L of oxygen via nasal cannula
Weaning with Tracheostomy
For patients with tracheostomy, follow the above weaning steps, then proceed with steps A-G below, ideally one day per step
A. Off ventilator for 5 minutes each hour, gradually increasing to 15 minutes. Nighttime TU/CPAP
B. Off ventilator for 20 minutes every two hours, gradually increasing to 40 minutes. Nighttime TU/CPAP
C. Off ventilator for 40 minutes every two hours, increase to 60 minutes. Try with speaking valve. Nighttime TU/CPAP
D. Off ventilator for 60 minutes every two hours, increase to 90 minutes. Try with speaking valve. Nighttime TU/CPAP
E. Off ventilator during daytime except for inhalations, to be given at least 4 times/day*. Nighttime TU/CPAP
F. Entire day (and night) off ventilator except for inhalations, to be given at least 4 times/day*
G. When the patient is judged to have full spontaneous breathing, consider decannulation, preferably in the morning
* If no inhalations are prescribed, order 4 ml NaCl 4 times daily as inhalation
Criteria for Decannulation
- Has managed an entire day without ventilator support, except for inhalations
- No ongoing clinical deterioration
- Retains swallowing function and can maintain a clear airway
- Can breathe past the tracheostomy tube and speak
Lung Recruitment
These maneuvers are rarely effective later than 48 hours after the onset of ARDS and may cause severe injuries. During any lung recruitment, circulation and pulse oximetry should be closely monitored. Lung recruitment has the best effect early in the course of airway collapse and development of acute lung failure.
Method 1
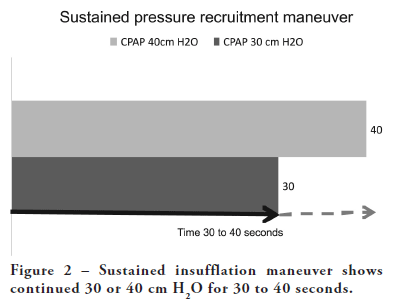
Insufflate a lung air-oxygen mixture to an airway pressure of 40–50 cm H2O for 20–30 seconds. Then continue normal ventilation, initially maintaining a PEEP of 15–20 cm H2O. The maneuver can be repeated after a few minutes if oxygenation does not improve. During and after insufflation, use the lowest oxygen concentration possible to minimize the development of absorption atelectasis. The PEEP level can then be slowly reduced if arterial oxygen saturation is maintained. This can be achieved by having the ventilator insufflate the lung for a long time, setting it to CPAP mode (continuous positive airway pressure) with a total pressure of 40–50 cm H2O, or by extending the inspiration time under pressure-controlled ventilation and setting the ventilator to a total peak pressure of 40–50 cm H2O. This maneuver can also be done manually with a resuscitation bag connected to a manometer. It is crucial that airway pressure remains at least at the PEEP level after the maneuver and is not allowed to drop to zero or become negative. Therefore, avoid airway suction at this stage if possible.
Method 2
Frequent lung insufflations to an airway pressure of 40–50 cm H2O over a period of 5–10 minutes. This is achieved by setting the ventilator to a low respiratory rate (10/min), a long inspiration time (50%), a PEEP of 20 cm H2O, and a total peak pressure (including PEEP) of 40–50 cm H2O, and ventilating with these settings for 5–10 minutes. Then, the ventilator is set to the settings outlined in Method 1.
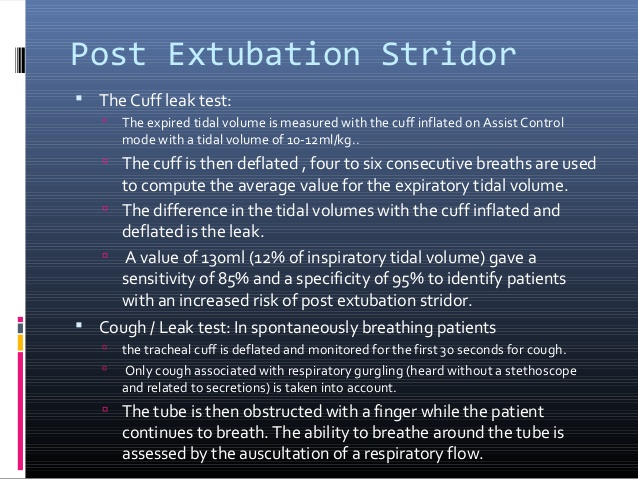
Extubation
Extubation is the removal of the endotracheal tube performed after anesthesia or intensive care with intubation and ventilator treatment. The tube is removed when the patient can breathe satisfactorily without becoming fatigued and without the risk of desaturation. Extubation of a fatigued patient is contraindicated. Acceptance of 5 over 5 in support pressure/PEEP with FiO2 below 0.3 is considered a safe measure for extubation. In some cases, higher pressure levels can be safely extubated. It is always an advantage if the patient is communicative and calm during extubation. “Crash extubation” should be avoided.
Criteria for Extubation
- Spontaneous eye opening
- Facial grimace
- Patient movement other than coughing
- Conjugated gaze
- Purposeful movements
- Endtidal levels of anesthetic gases lower than:
- Sevoflurane: 0.2%
- Isoflurane: 0.15%
- Desflurane: 1.0%
- Oxygen saturation higher than 97%
- Positive laryngeal stimulation test
- Endtidal volume greater than 5 ml/kg
Evaluate the Patient’s Ability to Breathe Spontaneously Before Extubation
- Can the patient receive supported ventilation in the ventilator?
- Are acceptable values present for TU, PEEP 10, FiO2 <40%?
- Are blood gases with SaO2 >95%, PaO2 >10 kPa, PaCO2 <6 kPa?
- Reduce to extubation settings with PEEP 5-7 and TU 5-7
Extubation Criteria
- Alertness RLS <3?
- Swallowing function, coughing strength?
- Handles PEEP 5-7 and TU 5-7 or free on nasal cannula > 30 minutes?
- Expected clear airway after extubation?
Evaluation After Extubation
- Pulse, blood pressure, respiratory rate, blood gases?
- Within individually set limits?
- Reminder: A pilot must plan both takeoff (intubation) and landing (extubation).
- Extubation is always elective (unless accidental).
- 23% of serious airway-related events occur during extubation.
- The most common complications during extubation are: hypertension, tachycardia, increased intracranial and intraocular pressure, etc.
- Common problems: inadequate oxygenation and ventilation. Inability to protect the airway and clear mucus from the airways.
- During extubation, consider that extubation may fail.
- During extubation, assess whether reintubation will be easy or difficult.
- Reintubation under optimal conditions differs greatly from emergency reintubation of a hypoxic patient.
- Consider delayed extubation.
- Consider extubation over an “Airway exchange catheter.” Pre-treat with inhalation of lidocaine.
- ARDS
- Recent tracheostomy
- COVID-19 infection
- Whooping cough (including previous whooping cough)
- Sarcoidosis
- COPD
- Restrictive lung diseases – lung fibrosis
- Tracheomalacia
- RS-virus pneumonia
- Lymphoma
- Lidocaine (Xylocaine) 10 mg/ml, 1 ml +
- Bupivacaine 5 mg/ml, 1 ml +
- NaCl 1 ml (total of 3 ml), in a nebulizer
- Glucose 5% 1000 ml +
- Theophyllamine (23 mg/ml) 20 ml +
- Bethametasone (Betapred) 4 mg
- Unconsciousness
- Carbon dioxide retention
- Skull base fracture
- CSF leak
- Nasal fracture
- History of nosebleeds
- Thrombocytopenia < 85
- Nasal obstructions
- Recent nasal surgery
- Mixer/flowmeter
- Humidifier Fisher & Paykel
- Tubing set with tube and moisture chamber
- Gas connection to the moisture chamber
- Sterile water
- Optiflow nasal cannula
- Optiflow tracheal connection
- Connect the mixer/flowmeter to the air and oxygen outlets.
- Prepare the tubing set and connect the moisture chamber to the sterile water.
- Attach the blue breathing tube to one outlet on the moisture chamber and the adapter to the other. The yellow-marked cable only heats the inspiration tube. The blue-marked cable measures the temperature in the inspiration tube.
- Turn on the humidifier
- The humidifier should be set to invasive ventilation mode; it cannot be set to an exact degree but adjusts automatically; chamber 35.5–42°C, airway 35–40°C. It will alert for low temp, <35.5°C, or high temp, >41°C.
- Set the desired oxygen concentration (FiO2) on the mixer.
- The flow from the mixer should be at least 20 liters per minute to provide the correct oxygen concentration. The maximum flow that can be set is 60 liters per minute. Thegoal of the high flow is to exceed the patient’s inhalation flow and therefore create minimal dilution with room air. This way, the patient will receive the set amount of oxygen.
- Connect the Optiflow nasal cannula/Optiflow tracheal connection to the blue humidifier tubing and attach it to the patient. Gradually increase the gas flow at startup until the humidifier reaches working temperature.
- Initiated by the patient and works exactly the same as pressure support, except that the pressure support level is set to zero
- Maintains positive airway pressure at all times
- Is essentially a spontaneous breathing method with continuous positive pressure to keep the airways open
- Automatically adjusts the breathing pattern smoothly to the patient’s condition, switching between active and passive breathing patterns.
- Machine-generated breaths are pressure-controlled.
- Spontaneous breaths are pressure-supported.
- Prevents tachypnea.
- Prevents auto-PEEP.
- Prevents dead space ventilation.
- Does not exceed a Pinsp pressure of 10 cmH₂O below the upper pressure limit.
- It is a time-cycled, pressure-limited mode that allows spontaneous breathing throughout the ventilation cycle.
- It has two pressure levels with time cycles and switches between these levels. The patient can breathe spontaneously at both levels, and pressure support can be provided at both levels.
- It is a time-cycled, pressure-limited mode that allows spontaneous breathing throughout the ventilation cycle.
- Alternates between two levels of positive airway pressure, with most of the time spent at the higher level and a brief exhalation to facilitate ventilation.
- Differs from Bi-Vent by using an inverted I:E ratio.
- An alternative ventilation mode when conventional strategies do not achieve the expected effect
- A possible alternative to prone positioning in severe lung failure
- An alternative lung recruitment method
- Hypoxic respiratory failure (type 1) with high PEEP requirement (≥ 12 cmH₂O) and low P/F ratio (≤ 26.7)
- Lower indication threshold with clear clinical deterioration
- Potentially recruitable low lung compliance
- Breathing dyssynchrony with conventional ventilator settings due to high or variable respiratory rate
- Obstructive lung disease (COPD, asthma with expiratory time constant >0.8 s)
- Right heart failure
- Severe hypovolemia
- High intracranial pressure
- Phigh in most cases between 25-30 cm H₂O
- As a basis, the average of Pmean and Ptop from the previous conventional ventilator setting can be calculated.
- Plow (marked as “PEEP”): 0 cm H₂O or the lowest value allowed by the ventilator
- Thigh: 4 seconds
- Tlow: 0.4 seconds
- I:E 10:1 (should not be considered fixed)
- All TU (pressure support): 0 cm H₂O
- Evaluate release volumes (the tidal volume measured during the release periods) and adjust Tlow to achieve release volumes of 6-8 ml/kgABW. Decrease Tlow in 0.05-second steps to reduce release volume or increase Tlow in the same increments to increase release volume.
- Before increasing Tlow, review the pressure and flow curves to avoid derecruitment, which could occur with excessively long Tlow. The shift from low to high pressure should occur at an expiratory flow that corresponds to approximately 50-75% of peak flow. If the flow falls below 50% of peak flow, auto-PEEP is lost, increasing the risk of derecruitment. If desired release volumes cannot be achieved without a dangerous flow reduction, increase Phigh by 2 cm H2O increments.
- Check a blood gas within 30 minutes.
- If the patient retains carbon dioxide, optimize sedation to improve spontaneous breathing effort. Decrease Thigh by 0.5-second increments to increase the frequency of release periods. To maintain Pmean, consider increasing Phigh by 1-2 cm H2O increments.
- To improve PaO2, increase Phigh by 2 cm H2O increments. If PaCO2 allows, you can also lengthen Thigh. An unreasonably long Thigh (>5 seconds) may not provide further oxygenation improvement and could be uncomfortable for the patient.
- Evaluate the patient’s breathing effort. If there is evident respiratory distress, try reducing Thigh in 0.05-second steps and adjust Phigh (either increase or decrease by 2 cm H2O) to find the optimal value. Ensure release volumes are sufficiently large. Cautiously deepen sedation to avoid losing spontaneous breathing entirely. If the patient continues to struggle against the ventilator despite all interventions, switch back to a conventional mode.
- Fredericks AS, Bunker MP, Gliga LA, et al. Airway Pressure Release Ventilation: A Review of the Evidence, Theoretical Benefits, and Alternative Titration Strategies. Clin Med Insights Circ Respir Pulm Med. Published 2020 Feb 5.
- Farkas J IBCC chapter: Guide to APRV for COVID-19, April 8, 2020, https://emcrit.org/pulmcrit/aprv-covid/
- van der Zee P, Gommers D. Recruitment Maneuvers and Higher PEEP, the So-Called Open Lung Concept, in Patients with ARDS. Crit Care. 2019;23(1):73. Published 2019 Mar 9.
- Jain SV, Kollisch-Singule M, Sadowitz B, et al. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp. 2016.
- Ehab G Daoud, Hany L Farag, Robert L Chatburn: Airway Pressure Release Ventilation: What Do We Know? Respiratory Care Feb 2012, 57 (2) 282-292
- With conventional settings and in the absence of spontaneous breathing, DuoPAP resembles Pressure Control Ventilation Plus (PCV+).
- If the frequency is reduced and Thigh is kept short compared to the low-pressure phase, this breathing pattern resembles PSIMV+ with spontaneous breaths after controlled breaths.
- If Thigh is set close to the breath cycle time with sufficient time at the lower level to allow full or nearly full expiration, this breathing pattern resembles APRV.
- Delivers a preset tidal volume or minute volume during a preset inspiration time and at a preset respiratory rate, regardless of changes in lung or chest resistance or compliance.
- Maintains constant flow with varying peak pressure.
- Synchronized Intermittent Mandatory Ventilation
- The user sets how many SIMV breaths (mandatory breaths) should be delivered each minute.
- The patient can breathe spontaneously with pressure support between mandatory breaths.
- The spontaneous/pressure-supported breath is defined by setting the pressure support level above PEEP.
- If the patient does not breathe at all, only the mandatory breaths are delivered.
- If the patient triggers during the SIMV period, the set mandatory breath is delivered. During the next SIMV period, the ventilator waits for patient triggering, but if this does not occur within the first 90% of the respiratory cycle time (SIMV period), a mandatory breath is delivered.
- Delivers a preset tidal volume or minute volume during a preset inspiration time and at a preset respiratory rate, regardless of changes in resistance or compliance in the lung or chest.
- Maintains constant flow with varying peak pressure.
- Delivers a constant pressure during a preset inspiration time and at a preset respiratory rate.
- Delivers inspiration with a decelerating flow.
- Changes in lung or thoracic resistance or compliance affect the delivered volume.
- Initiated by the patient, who controls breathing rate and tidal volume.
- Delivers ventilator support at the preset pressure level with a decelerating flow.
- Provides backup ventilation (PCV) in case of apnea.
- Initiated by the patient, who controls their breathing rate.
- Provides ventilator support with a variable peak pressure and a decelerating flow to guarantee the preset tidal volume.
- The inspiratory pressure in a breath will never exceed 5 cm H2O below the upper pressure limit.
- The ventilator provides backup ventilation (VCPS) in case of apnea.
- VCPS combines the advantages of volume control and pressure control by delivering a preset tidal volume with decelerating inspiratory flow at a preset respiratory rate.
- Maintains the lowest possible constant pressure throughout inspiration.
- The inspiratory pressure in a breath will never exceed 5 cm H2O below the upper pressure limit.
- Is an interactive mode that automatically switches between controlled mode (VC) and supported mode (VS) based on patient triggering.
- Delivers controlled breaths in the absence of patient breathing attempts but switches to supported breathing when a breathing attempt is detected.
- Works as a support tool at the beginning of the weaning period.
- Adjusts according to the patient’s breathing capacity.
- Is an interactive mode that automatically switches between controlled mode (VCV) and supported mode (VS) based on patient triggering.
- Delivers controlled breaths in the absence of patient breathing attempts but switches to supported breathing when a breathing attempt is detected.
- Works as a support tool at the beginning of the weaning period.
- Adjusts according to the patient’s breathing capacity.
- Is an interactive mode that automatically switches between controlled mode (PC) and supported mode (PS) based on patient triggering.
- Delivers controlled breaths in the absence of patient breathing attempts but switches to supported breathing when a breathing attempt is detected.
- Works as a support tool at the beginning of the weaning period.
- Adjusts according to the patient’s breathing capacity.
- Smaller lung volume/kg, larger anatomical dead space, and greater breathing effort compared to adults.
- Higher metabolism = higher O2 consumption.
- Normal expiration at rest causes the lungs to fall below their closing capacity.
- Lack of bronchioalveolar connections (increased risk of atelectasis).
- Young children desaturate quickly during apnea.
- Preoxygenation may be difficult during induction.
- You rarely have time to search for the right equipment when things get serious…
- Usually pressure-controlled ventilation for children (PCV, PSV, or VCPS are the most common modes).
- Tidal volume is typically 6-7 ml/kg, PEEP 5 as standard (sometimes higher).
- Preferably FiO2 < 0.5.
- Recruitments can (and should) be done as usual, but often do not yield as good results as in adults.
- How much leakage from an uncuffed tube is tolerable is a debated topic…
- NAVA ventilation is used sometimes (perhaps too rarely).
- Can be used with a full mask, nasal mask, “dummy plug,” or nasal tube.
- Full masks work best on older children; nasal masks or dummy plugs on younger ones.
- Nasal tubes can work but often bother patients, leading to a lot of fussing and poor breathing. Best used if the patient transitions from nasal intubation to NIV.
- NIV has become less common in pediatric ICUs since the advent of high-flow nasal cannulae.
- Requires competent nurses to function effectively.
- Young children need to be quite awake to avoid apnea after extubation.
- Preferably PEEP 5 and TU 5-7, FiO2 < 0.35.
- Suction the oropharynx and clear the nostrils, provide nasal drops if necessary.
- Solu-Cortef 5 mg/kg (max 100 mg) can be given if the patient has been on a ventilator for a few days.
- Micronefrin (racemic adrenaline) in a nebulizer works well for smaller children to reduce upper airway swelling, 0.05 ml/kg, max 0.75 ml (not in the tube or tight mask).
- High-flow nasal cannulae are very useful if you anticipate the patient will have difficulty after extubation.
When administering anesthesia to patients, one often has the choice between general anesthesia or some type of regional block, central or peripheral, combined with sedation for the surgical procedure. It may seem tempting to choose regional anesthesia for patients with difficult airways, but one must choose a variant that “surely works,” such as spinal anesthesia. If a block stops working during surgery and one is forced to quickly induce general anesthesia in a patient with a difficult airway, serious problems can arise.
Coughing – During Ventilator Treatment
Severe persistent coughing can be a serious issue during the treatment of intensive care patients on a ventilator and during weaning. Severe coughing is commonly seen in conditions such as:
Severe coughing in a ventilated patient can be treated experimentally with nebulizer inhalation (3-6 times a day) of:
This treatment can provide significant cough suppression for a few hours and can be given 3 to 6 times daily. An injection of lidocaine (Xylocaine) 50 mg intravenously before mobilization can also be effective. Intravenous opioids can also offer some cough suppression. For bronchospasm and coughing, a so-called asthma drip may be tried, administered over 12 hours:
HFNC – High-Flow Nasal Cannula – Optiflow
Optiflow (HFNC) is a type of breathing support for spontaneously breathing patients that allows for airway inflation with resistance breathing without a tight-fitting mask. Optiflow often provides better comfort and less effort for the patient compared to CPAP with a tight-fitting mask. Optiflow uses oxygen at a very high flow rate, 20-60 L/min with active humidification (NHF – nasal high flow). The system can be connected via either a nasal cannula or a tracheal connection. It allows for comfortable and effective delivery of up to 100% inhaled oxygen.
Background
A high oxygen requirement almost always leads to dry airways and impaired mucus transport. Active humidification of the oxygen flow can be used during spontaneous breathing when there is a high oxygen requirement but no need for CPAP or NIV pressure. Active humidification allows for high inhaled oxygen concentrations without drying out the airways. Gas flow above 20 liters per minute also creates a slight positive airway pressure (low), and washes out exhaled gas from the nose/throat, minimizing re-inhalation of carbon dioxide. The desired oxygen concentration is regulated using the gas mixer.
Indications
This method can be used instead of an oxygen mask when the goal is to avoid drying out the upper airways. Examples include switching between NIV and mask, or during long-term oxygen needs. Gas flows up to 35 liters per minute can be used without a doctor’s order. Higher flows are used only with the approval of the responsible physician. Weaning from non-invasive ventilator treatment can be done as effectively with Optiflow as with a tight-fitting mask (NIV).
Contraindications
Practical Instructions
Equipment
Optiflow via Nasal Cannula
Optiflow via Tracheal Connection
Active humidification via Optiflow tracheal connection with high flow is a good option for patients with tracheostomy who are intermittently breathing spontaneously, such as during ventilator weaning.
The blue cup is bowl-shaped to catch expectorated secretions. It can be removed and cleaned with sterile water. The system is set up in the same way as described above.
Speaking Valve
Patients with a speaking valve have very limited ability to humidify their airways. By connecting an Optiflow nasal cannula while using the speaking valve, the patient can receive good humidification with oxygen. Therefore, oxygen via the speaking valve can be omitted.
Replacement/Cleaning
The sterile water bag should be replaced once per day. The tubing/cannula/tracheal connection is single-patient use and should be replaced once per week. Clean the cannula as needed with sterile water.
Troubleshooting
If the humidifier does not reach the set temperature, drain any condensation at the temp probes and check that the tubing is warm. Ensure the temp probe is inserted from above and pushed in fully!
LED lights show what has triggered an alarm. Tip: Hold down the humidifier’s “mute alarm” button to check the temperature at several points.
CPAP – Continuous Positive Airway Pressure
Increased resistance breathing. Requires spontaneous breathing and that the patient triggers each breath. Can be done with the patient in a ventilator or with a closed breathing mask with a pressure valve. Can be combined with pressure-supported breaths in a BiPAP system or CPAP with ASB. A pressure higher than atmospheric pressure is maintained throughout the breathing cycle (exhalation + inhalation + pause). CPAP makes inhalation easier but exhalation more difficult. The term CPAP (Continuous Positive Airway Pressure) is commonly used for spontaneously breathing patients.

The CPAP system provides high airflow (80-100 L/min) with constant pressure and flow via mask ventilation. The system can compensate to some extent for leaks in mask breathing. It recruits lung tissue and increases oxygenation. It is mainly used when breathing strength is retained during pulmonary edema or atelectasis. CPAP can be dangerous if breathing strength is insufficient. The advantage is that it keeps the airway open and increases FRC, which makes breathing easier. However, CPAP is demanding for the patient over time and can lead to fatigue. In cases of pneumonia, it can cause respiratory collapse. Effective against pulmonary edema.
CPAP normal settings
| Normala settings in CPAP | |
|---|---|
| CPAP-level | 3-10 cm H2O |
| RR: | Varying |
| FiO2: | 30-100% |
PEEP – Positive End-Expiratory Pressure
Increased resistance in exhalation for intubated patients via a valve in the ventilator. PEEP maintains positive airway pressure at all times. PEEP recruits lung tissue and improves oxygenation by preventing the airway pressure from dropping back to atmospheric pressure at the end of expiration. PEEP is usually used in combination with other ventilatory modes and serves as a basic setting in all ventilator modes. The PEEP valve controls the exhalation valve via servo control. It is primarily used for pulmonary edema or atelectasis.
PEEP can lead to impaired venous return and reduced hemodynamics, resulting in blood pressure drops. It can also cause increased brain swelling and should be limited in cases of elevated intracranial pressure. PEEP is most effective in extrapulmonary causes of respiratory failure, such as obesity or heart failure, and works best early in the course of severe lung disease. Typical PEEP levels are 5-10 cm H2O, but in ARDS, higher pressures of 10-20 cm H2O may be needed. Higher PEEP is more effective with diffuse infiltrates than localized ones. Excessive PEEP can overdistend the lungs, causing damage and potentially leading to pneumothorax in severe cases. If high PEEP leads to increasing PCO2, the lungs may be overdistended, impairing gas exchange.
PEEP level: 3-20 cm H2O.
Ventilator settings in different degrees of pulmonary injury
| Ventilator settings | Healthy lung | Moderate pulmonary failure | Severe pulmonary failure |
|---|---|---|---|
| Breathing mode | PS, VC, VCPC, BiPAP | PS, VC, PC, VCPC, BiPAP | PC, (PS, VCPC), BiPAP |
| Tidal volume ml/kg | < 6 - 8 | < 6 - 8 | < 6 - 8 |
| Respiratory rate | 15 - 20 | 15 - 20 | 15 - 30 |
| I:E quote | 1:2 | 1:2 - 1:1 | 1:1 - (2:1) |
| PEEP cm H20 | 0 - 5 | 5 - 10 | 10 - 20 |
| Oxygen fraction % | < 40 | 40 - 60 | 40 - 100 |
| Lung recruitement | - | Yes | Yes, at an early stage |
ASV – Adaptive Support Ventilation
ASV stands for Adaptive Support Ventilation, a mode in Hamilton ventilators. ASV maintains a user-defined minimum minute ventilation regardless of the patient’s breathing pattern. The target for the breathing pattern (tidal volume and respiratory rate) is calculated by the ventilator based on the pattern that minimizes breathing effort and provides the lowest possible ventilator-generated pressure when the patient is not making any breathing efforts.
ASV guarantees a selected minute volume through pressure control. It allows spontaneous breathing and adjusts ventilator support to the patient’s needs, so no change in mode is required if the patient starts or stops triggering. Minute volume is set by selecting a value for “%MinVol”; 100% corresponds to 100 ml/min/kg ideal body weight for adults and 200 ml/min/kg for children. By choosing a value other than 100%, the desired minute volume can be set.
Start settings in ASV ventilation
| Patient group | Ideal Weight (kg) | Pinsp (cm H2O) | TI (sec) | Initial frequency (b/min) | Min. Target Frequency (b/min) |
|---|---|---|---|---|---|
| Pediatric patient | 15-20 | 15 | 0.8 | 20 | 10 |
| 21-23 | 15 | 0.9 | 15 | 7 | |
| 24-29 | 15 | 1 | 15 | 7 | |
| > 30 | 15 | 1 | 15 | 7 | |
| Adults | 30-39 | 15 | 1 | 14 | T |
| 40-59 | 15 | 1 | 12 | 6 | |
| 60-89 | 15 | 1 | 10 | 5 | |
| 90-99 | 18 | 1.5 | 10 | 5 | |
| > 100 | 20 | 1.5 | 10 | 5 |
ASV adjusts the inspiratory pressure and respiratory rate breath by breath, accounting for changing airway pressures (resistance, compliance, RC-exp) and uses lung-protective settings to reach the targets. A reduction in pressure limitation is followed by a decrease in tidal volume (TV) and an increase in frequency.
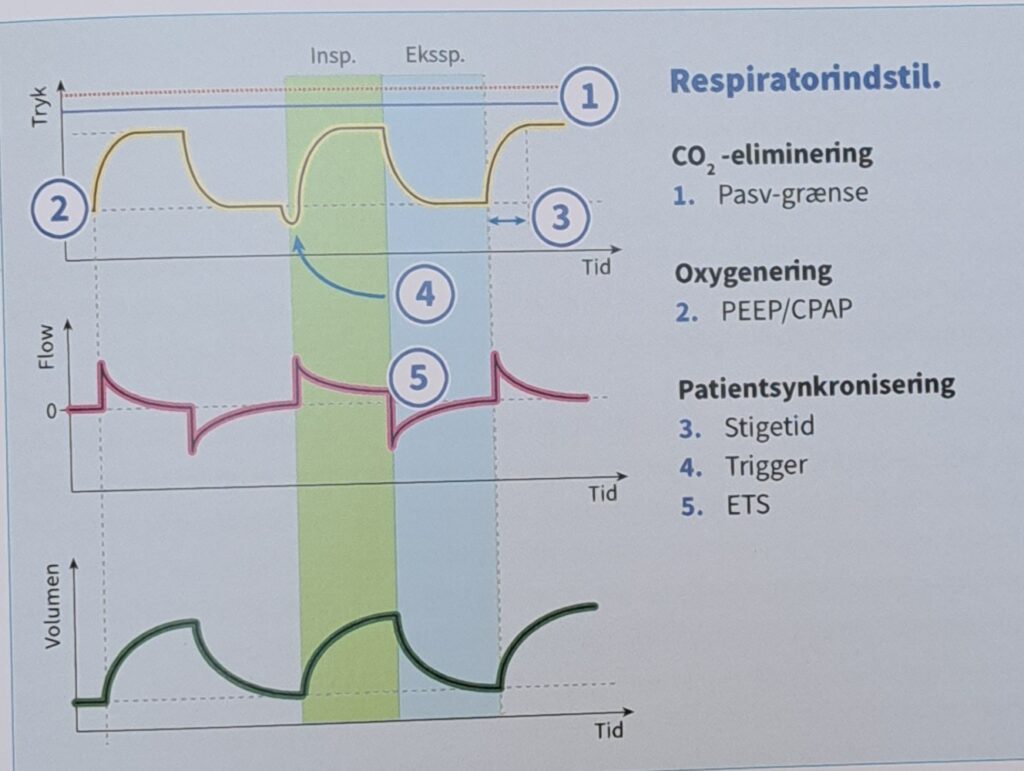
ASV maintains a preset minimum minute ventilation:
The user sets “Min.Vol,” “PEEP,” and “Oxygen (FiO2).” More information on working with ASV can be found in the Hamilton Medical ventilator manual.
Practical Information on Working with ASV
Hamilton recommends setting the alarm limit for “High Pressure” to at least 25 cmH₂O above PEEP/CPAP. The maximum peak pressure delivered in ASV (Pasv limit) is 10 cmH₂O below the alarm limit.
Connect the patient to the ventilator and start ASV. The ventilator begins with three test breaths. It automatically selects values for respiratory rate (fTotal), inspiratory time (TI), and inspiratory pressure (Pinsp) based on the calculated IBW.
Once ASV starts, the ventilator calculates an optimal breathing pattern and sets target values for tidal volume and frequency according to ASV rules, specifying “Min.Vol” to achieve the goals. Depending on whether the patient is passive or active, the ventilator delivers volume-controlled or pressure-supported breaths using a lung-protective strategy. More details are available in the Hamilton Medical ventilator manual. Once the calculated goals are achieved, ventilation results should be assessed. All monitored airway parameters can be used for this purpose. To evaluate acid/base status, continuous arterial blood gas monitoring is recommended, and minute ventilation should be adjusted accordingly.
Examples of adjustments during ASV ventilation
| Condition | Change in Minute Volume |
|---|---|
| Normal arterial blood gases | None |
| High PetCO₂, or PaCO₂, | Increase 'Min.vol'. Pay attention to the inspiratory pressure. |
| Low PaCO₂ | Reduce 'Min.volume'. Pay attention to mean pressure and oxygenation status. |
| High respiratory drive | Consider increasing 'Min.Volume' Consider sedation, analgesia or other treatments |
| Low 0₂ saturation | No. Consider increasing PEEP/CPAP and/or oxygen levels. |
Weaning from ASV
This section does not provide clinical information beyond what is necessary for using ASV. ASV always allows the patient to breathe spontaneously. Periods of spontaneous breathing can occur and are supported by ASV, even during periods of fully controlled ventilation. This means weaning can begin with ASV before it can be clinically detected. Therefore, monitoring the patient’s spontaneous breathing pattern over time is important.
Progress in weaning can be tracked in the trend display by looking at inspiratory pressure (Pinsp), total frequency (fTotal), and spontaneous frequency (fSpont). It may be necessary to reduce the “Min.Vol” setting to 70% or lower to “encourage” the patient to resume spontaneous breathing. If the patient can maintain several minutes or even hours at a low “%Min.Vol” setting, it does not mean the weaning is complete. The “Min.Vol” setting must always be interpreted together with the “Pinsp” level needed to achieve the set minute ventilation. Only if “Pinsp” and “fControl” are at their lowest values can weaning be considered complete.
BiPAP – Biphasic Positive Airway Pressure
Pressure-controlled ventilation with room for spontaneous breathing throughout the entire breathing cycle. Adjustable pressure support in two modes. Pressure levels are set for both CPAP breaths and assisted breaths (ASB). BiPAP improves alveolar ventilation and increases functional residual capacity (FRC) while keeping the airway open. BiPAP is used to treat mild, moderate, and severe lung failure.
BiPAP-assist (with ASB)
Pressure-controlled assisted ventilation.
BiPAP Ventilator Settings
| BiPAP | Normal settings in the ventilator |
|---|---|
| ASB: | 3-10 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 10-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
| CPAP: | 3-7 cm H2O |
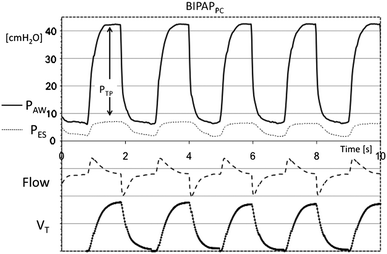
(S)CMV+
Corresponds to VCPS with automode. Pressure control with set tidal volume as the target. If the patient triggers, a volume-controlled breath is delivered corresponding to the set tidal volume with the lowest possible pressure.
APRV – Airway Pressure Release Ventilation
Spontaneous breathing at two different pressure levels, which are regulated independently in patient-controlled respiratory ventilation. APRV improves alveolar ventilation and increases functional residual capacity (FRC) while keeping the airway open. APRV provides CPAP at two different levels during complete spontaneous breathing and is used to treat both moderate and severe lung failure. APRV is available in Dräger ventilators as a specific ventilation mode.
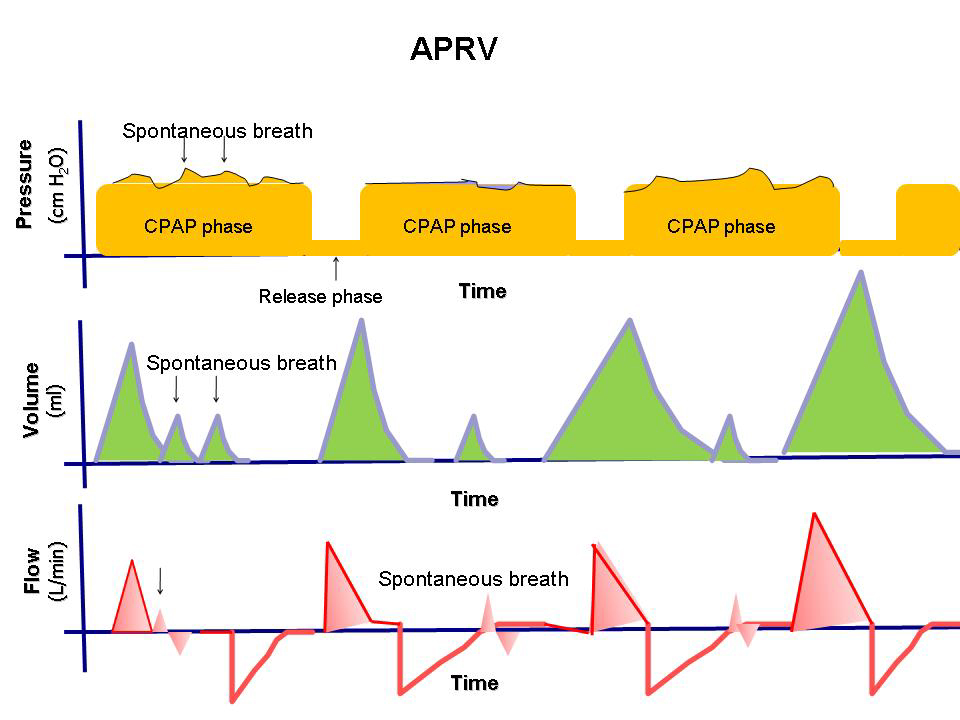
Simply put, APRV is a ventilator mode that allows spontaneous breathing with high CPAP resistance, which is varied with brief pressure releases in the breathing cycle to improve overall carbon dioxide elimination from the lungs.
APRV can be used as:
Indications
Contraindications
All contraindications are relative and must be carefully evaluated on a case-by-case basis.
Initial Settings
Adjustment of Settings
Weaning
If there is an overall clinical improvement (FiO2 <50%, calm spontaneous breathing [10-25 breaths/min] in a lightly sedated patient [RASS -1/-2]), start by reducing Phigh in 2 cm H2O increments. When Phigh drops below 20 cm H2O, extend Thigh in 0.5-1-second increments every 4-8 hours. The goal is to bring the patient closer to true CPAP treatment.
Theoretical Background
The APRV mode conceptually differs from other ventilation modes, focusing on slowly recruiting and keeping the lungs open. The long high-pressure phase allows the alveoli to fill uniformly, reducing heterogeneity in lung inflation. The patient’s preserved spontaneous breathing during Phigh improves V/Q matching and mimics physiological intrathoracic pressure changes, benefiting hemodynamics. With lower peak pressures, there are fewer iatrogenic injuries. Typically, after a few hours of APRV settings, blood gas values improve, allowing a reduction in FiO2, but this requires sufficient time to see the effects of APRV treatment.
References
APRV Settings in the ventilator
| APRV settings in the ventilator | |
|---|---|
| TV: | 6-8 ml/kg |
| RR: | 10-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
| CPAP | 5-15 cm H2O |
| ASB | 8-15 cm H2O |
DuoPAP Breathing Pattern
DuoPAP is a form of ventilation where spontaneous breathing is delivered at two alternating CPAP levels. In this breathing pattern, the ventilator regularly alternates between two defined pressure levels of positive airway pressure or CPAP.
The transition between levels is regulated by the DuoPAP timing setting or by the patient’s own breathing efforts. In DuoPAP, the switch between the two levels is based on pressure settings (Phigh) and ‘PEEP/CPAP’, as well as timing (Thigh) and ‘Frequency’. Thigh corresponds to the duration of the high-pressure phase in the breathing cycle.
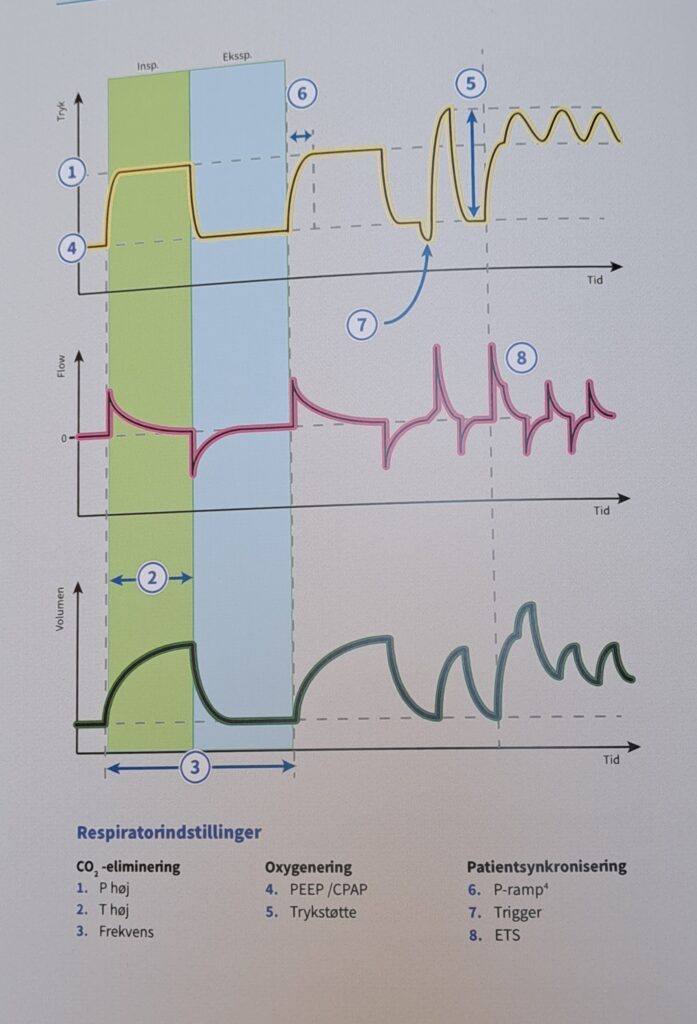
Important Notes:
‘Pressure Support’ can be used to assist spontaneous breathing in DuoPAP, regardless of whether it occurs at ‘PEEP/CPAP’ or ‘Phigh‘. ‘Pressure Support’ is set above ‘PEEP/CPAP’, meaning that spontaneous breaths are only supported at the ‘Phigh‘ level when this targetpressure is higher than ‘Phigh‘.
IPPV – Intermittent Positive Pressure Ventilation
IPPV keeps the lungs expanded by continuously inflating them with air. A constant volume is delivered in both controlled and assisted modes. Positive pressure is controlled by stimulating the patient’s spontaneous inhalation or by fully mechanical ventilation. Access to the patient’s lungs is either through intubation or a breathing mask. IPPV includes CPPV (Continuous Positive Pressure Ventilation), PLV (Pressure Limited Ventilation), Auto-flow (automatic regulation of inspiratory flow), and IRV (Inversed Ratio Ventilation). IPPV is mainly used in treating mild or moderate lung failure.
IPPV normal settings
| IPPV | Intermittent Positive Pressure Ventilation |
|---|---|
| ASB: | 3-12 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 10-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
| CPAP: | 3-15 cm H2O |
MMV – Mandatory Minute Ventilation
MMV delivers spontaneous breathing with automatic adjustment of mandatory breaths to meet the patient’s volume needs. Access to the patient’s lungs is through intubation or a breathing mask. MMV allows for PLV (Pressure Limited Ventilation) and Auto-flow (automatic regulation of inspiratory flow). MMV is mainly used in treating mild or moderate lung failure.
MMV Standard settings in the ventilator
| MMV - Mandatory Minute Ventilation | Normal settings |
|---|---|
| ASB: | 3-12 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 10-20 breaths/minute |
| TU: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
| CPAP: | 3-15 cm H2O |
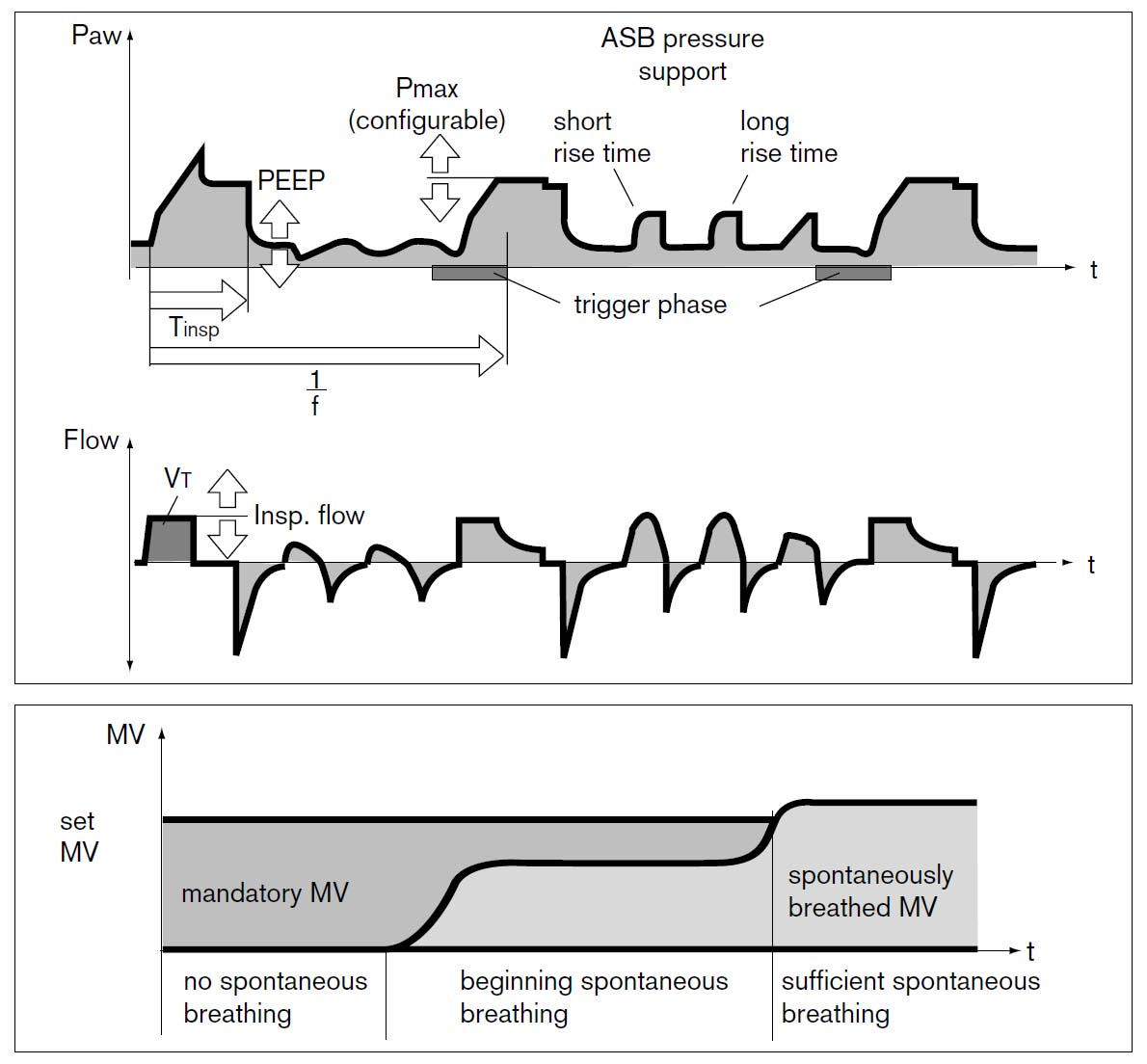
PCV+
Corresponds to Pressure Control with automode. If the patient triggers, a pressure-controlled breath is delivered based on the set PEEP and Pcontrol (pressure above PEEP).
SIMV – Synchronized Intermittent Mandatory Ventilation
Semi-controlled breathing where the patient does not need to breathe independently. SIMV is a combination of controlled and assisted ventilation modes. It provides a guaranteed preset number of breaths per minute, allowing spontaneous breathing between breaths. In SIMV, both the patient’s supported spontaneous breathing and the ventilator’s mandatory, controlled breaths are delivered. The breaths the patient initiates are either delivered with decelerating or constant flow, meaning volume-controlled or pressure-controlled breaths. SIMV is primarily used for ventilator weaning and in patients with good spontaneous breathing, often in the transition phase to spontaneous breathing. It can also be used for patients who do not tolerate volume-controlled or pressure-controlled ventilation. SIMV is used to treat both moderate and severe lung failure and in irregular breathing or when the patient is about to take over breathing independently. SIMV can be set with assisted breathing in VCPS mode or PCV mode.
SIMV ASB (Assisted Spontaneous Breathing)
SIMV Standard Settings in the Ventilator
| SIMV - mode | Normal settings |
|---|---|
| ASB: | 3-12 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 5-10 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
| CPAP: | 3-7 cm H2O |
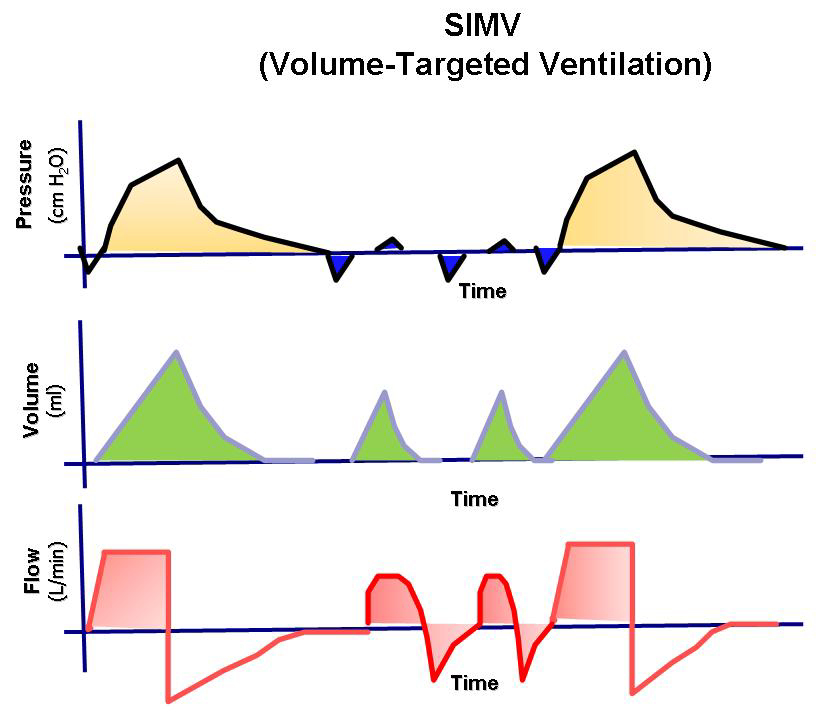
PSIMV+
Pressure-controlled mode. If the patient triggers between SIMV breaths, a pressure-supported breath is delivered.
VCV – Volume Controlled Ventilation
Controlled, predetermined breathing with positive pressure ventilation; the patient does not need to breathe independently. The volume of each breath is predetermined. It always provides a constant flow of inhaled air. It generates higher peak pressures in inhalation (end-inspiratory) compared to pressure-controlled ventilation, reducing the risk of hypoventilation. In volume-controlled ventilation, the peak pressure is higher than the plateau pressure. VCV is mainly used in treating mild or moderate lung failure. In flow/volume-oriented breathing modes, a constant inspiratory volume is maintained. These modes allow for extra flow to be triggered upon request during inspiration, so additional breaths can be triggered between normal breaths if the set trigger criterion is met.
VCV: Volume Controlled Ventilation - standard settings in the ventilator
| Respiratory physiology parameters | Normal settings |
|---|---|
| PS: | 5-15 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 12-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-15 cm H2O |
| FiO2: | 25-40% |
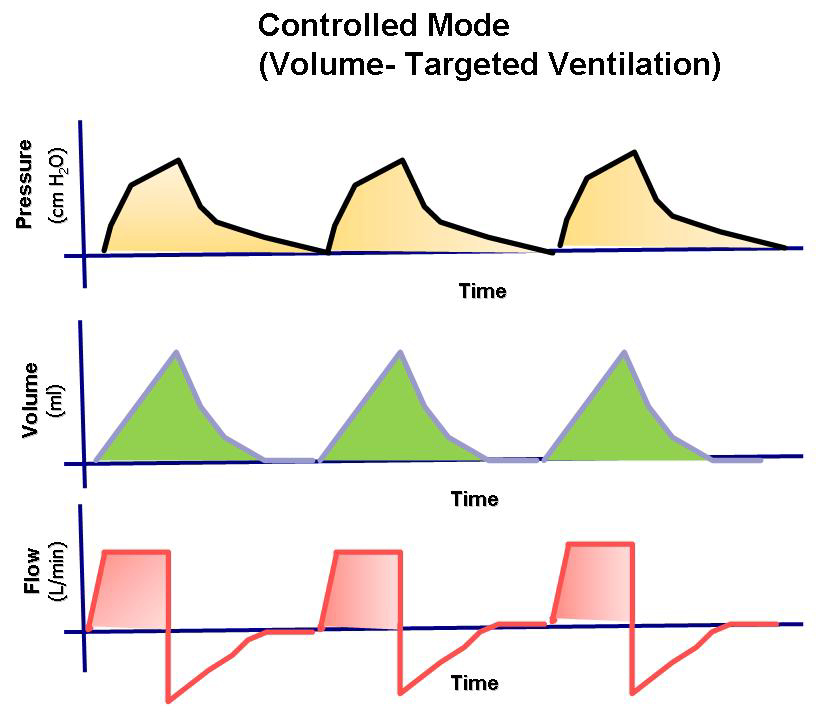
PCV – Pressure Controlled Ventilation
Controlled breathing where the patient does not need to breathe independently. The ventilator provides breaths with a constant preset pressure and decelerating flow during a preset inspiration time and at a preset respiratory rate. It always delivers a decelerating flow of inhaled air. It generates lower peak pressures compared to volume-controlled ventilation, reducing the risk of pressure-related lung injuries, which can be beneficial in ARDS. In pressure-controlled ventilation, the peak pressure equals the plateau pressure. Pressure-oriented modes maintain a constant preset pressure level during inspiration (pressure control, pressure support). PCV is mainly used to treat moderate or severe lung failure.
PCV - Pressure Controlled Ventilation - Standard Settings
| Respiratory physiology parameters | Normal settings |
|---|---|
| PS: | 5-15 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 12-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-15 cm H2O |
| FiO2: | 25-40% |
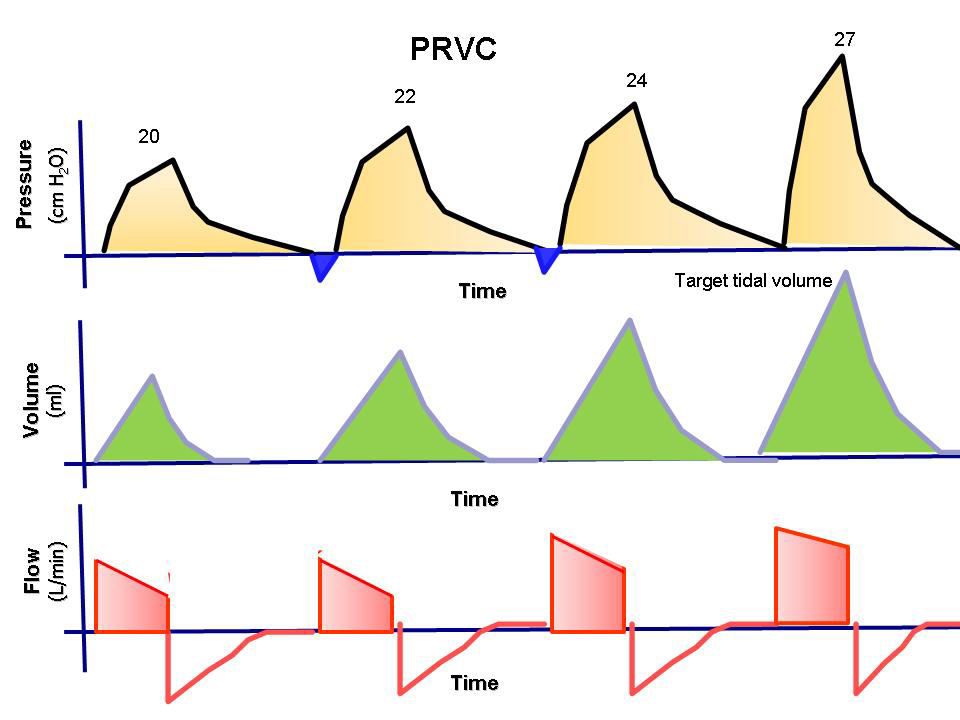
PSV – Pressure Supported Ventilation
Requires spontaneous breathing and that the patient triggers each breath independently. The ventilator provides breaths with a constant pressure throughout the inspiration period. All breaths are patient-triggered. It provides a decelerating flow of inhaled air. It delivers the peak pressure as set. This mode reduces the risk of pressure-induced lung injuries, making it beneficial in ARDS. In pressure-supported ventilation, the peak pressure equals the plateau pressure. This mode is mainly used during ventilator weaning and when spontaneous breathing is well established. It supports maintaining a constant set pressure during inspiration (pressure control, pressure support). TU is used to treat mild, moderate, and severe lung failure.
PS - Pressure Support Standard Settings
| Respiratory physiology parameters | Normal settings |
|---|---|
| PS: | 5-15 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 12-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
VSV – Volume Supported Ventilation
VCPR – Volume Controlled Pressure Regulation
VCPS ensures controlled breathing where the patient does not need to breathe independently. It delivers a decelerating flow of inhaled air. It sometimes results in higher peak pressure compared to pressure-controlled ventilation, reducing the risk of hypoventilation. It regulates the pressure so that the tidal volume remains constant and adjusts the pressure to deliver the lowest necessary pressure. VCPS is used to treat mild, moderate, and severe lung failure.
VCPS - Volume Controlled Pressure Support
| Respiratory physiology parameters | Normal settings |
|---|---|
| PS: | 5-15 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 12-20 breaths/minute |
| I:E | 1:2 |
| PEEP: | 5-15 cm H2O |
| FiO2: | 25-40% |
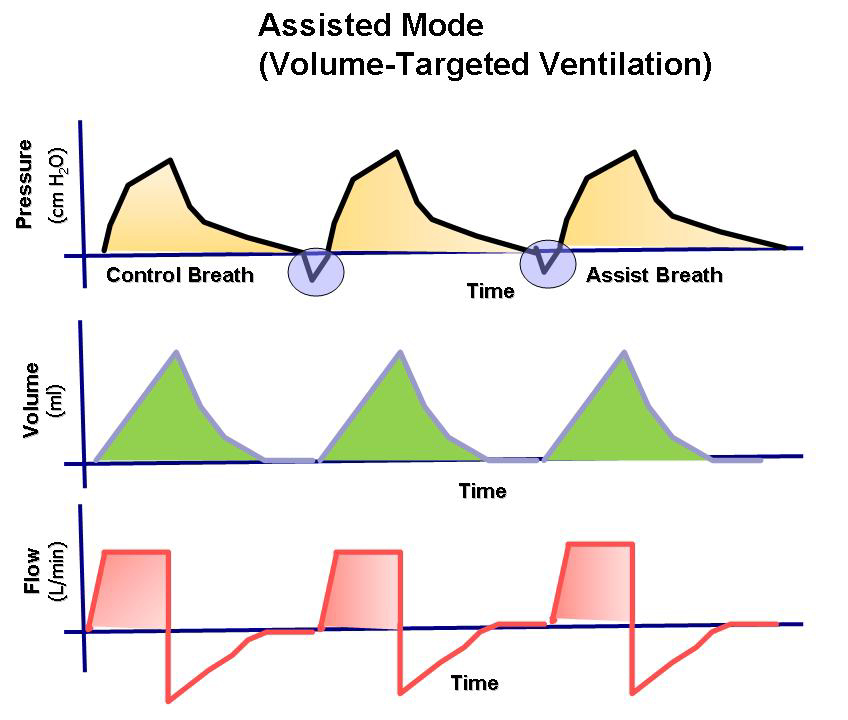
Automode VCPS – VS
Automode VC – VS
Automode PC + PS
NAVA – Neurally Adjusted Ventilatory Assist
There are four main settings in NAVA: NAVA level, PEEP, O2 concentration, and Edi trigger. In the ventilator, set the NAVA level (cmH2O/μV), PEEP (cm H2O), oxygen concentration (%), and Trigger Edi (μV). The default setting for Trigger Edi is 0.5 μV (0.1 – 2 μV). In pressure support, set the trigger sensitivity, inspiratory cycle (off), and pressure support above PEEP.
NAVA Ppeak = NAVA level x (Edi peak – Edi min) + PEEP. The estimated NAVA peak is displayed on the ventilator screen.
NAVA - Standard Settings
| Respiratory physiology parameters | Normal settings |
|---|---|
| NAVA level | 1,5-2,0 cm H2O/μV |
| Trigger Edi | 0.5 μV |
| PEEP: | 5-10 cm H2O |
| FiO2: | 25-40% |
Ventilator Treatment for Children
Ventilation Settings
NIV (Non-Invasive Ventilation)

Ventilation – Tips Before Extubation
Maquet/Siemens Ventilators (Getinge)
Servo-I Maquet, Siemens Servo 300, Siemens Servo 900 C. Siemens has been acquired by other companies, Getinge Group, and Maquet. The Servo-I is made by Maquet.
Maquet and Siemens Servo Ventilators Use Controlled and Supported Breathing Modes
Volume-controlled ventilation (VCV), pressure-controlled ventilation (PCV), volume-controlled pressure regulation (VCPS), pressure-supported ventilation (PS), and SIMV (Synchronized Intermittent Mandatory Ventilation). Servo ventilators feature Automode, improved volume support, new SIMV combinations (synchronized intermittent mandatory ventilation), and Open Lung Tool. Siemens Servo 900 C has volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) as its base. The Servo-I (Maquet) has volume-controlled pressure regulation (VCPS) as its base but also provides VCV, PCV, and several other breathing modes. The ventilator can be used for precise measurements of controlled, supported ventilation, or spontaneous breathing/CPAP.
Servo-I Display View
| Maquet/Siemens (Getinge) ventilators, | Display Settings |
|---|---|
| Pulmonary Airway Pressure (Paw): | Peak Pressure, Plateau Pressure, Airway mean pressure , PEEP |
| Minute volume (MV): | Inspiratory MV, Expired MV, Spontaneous MV, |
| Tidal volume (VT): | Inspired TV, Expired TV |
| Respiratory Rate (RR): | RR , Spontaneous RR |
| Breathing Work (WOB): | WOB in patient & ventilator, Shallow breathing index (SBI) |
| O2-concentration: | Inhaled O2- Fraction (FiO2) |
| Time Measures: | Inspiration-/Expiration (I:E), Inspiration-/tot. Respiratory time (TI/TTOT), Time constant (TC) |
| Lung mecanics: | Respiratory restistance, Compliance (C) static (in the respiratory system), Dynamic elastanse (E) |
| Air temperature: | 10-40 °C |
| Configuration of Curves: | Airway Pressures vs Time, Flow vs time, Volume vs time, CO2 vs Time |
| Capnography: | End-tidal CO2-conc. (etCO2), Minute elimination of CO2, Tidal CO2 elimination (VTCO2) |
| Trends: | MV, TV, RR, PEEP, R, C, etCO2, Peak-, plateau & mean pressures |
| Loops: | Paw vs Vol, Flow vs Vol. |
| Oxygen Saturation (SpO2): | SpO2, Pulse |
| Maquet/Siemens (Getinge) Ventilators | Normal Settings |
|---|---|
| PS: | 5-15 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 5-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-15 cm H2O |
| FiO2: | 25-40% |
| ASB: | 3-12 cm H2O |
| CPAP: | 3-10 cm H2O |
Aisys (ADU)(Datex-Ohmeda – GE Healthcare)
ADU, Aisys Carestation
The Aisys system offers several different breathing modes, including volume-controlled ventilation (VCV), pressure-controlled ventilation (PCV), pressure-supported ventilation (PSVPro (Pro=protection)), SIMV (Synchronized Intermittent Mandatory Ventilation, SIMV-VC, SIMV-PC), PCV-VG (pressure-controlled ventilation with volume guarantee), and CPAP/PSV. Aisys features improved volume support and various new SIMV combinations (synchronized intermittent mandatory ventilation). The Aisys system has volume-controlled ventilation (VCV) as its base but also offers PCV (PCV-VG) and other selectable breathing modes. The ventilator can be used for precise measurements of controlled, supported ventilation or spontaneous breathing/CPAP-PSV. The ventilator’s air pressure is located on the back of the breathing system. A precision valve controls gas flow to the patient. During inspiration, this gas flow closes the exhalation valve and pushes down the bellows. During expiration, a small flow presses the exhalation membrane to provide PEEP pressure.
ADU Aisys Care Stations Display Normal Settings
| ADU Aisys Carestation ventilator | Display Settings |
|---|---|
| Pulmonary Airway Pressure (Paw): | Peak Pressure,"Plateau Pressure", Airway mean pressure , PEEP |
| Minute volume (MV): | "Inspiratory MV ", Expired MV, Spontaneous MV |
| Tidal volume (VT): | Inspired TV, Expired TV |
| Respiratory Rate (RR): | "RR","Spontaneous RR " |
| Breathing Work (WOB): | WOB in patient & ventilator, Shallow breathing index (SBI) |
| O2-concentration: | Inhaled O2- Fraction (FiO2) |
| Time Measures: | Inspiration-/Expiration (I:E), Inspiration-/tot. Respiratory time (TI/TTOT), Time constant (TC) |
| Lung mecanics: | Respiratory restistance,"Compliance (C) static (in the respiratory system)", Dynamic elastanse (E) |
| Apnéa Time | |
| Air temperature: | 10-40°C |
| Configuration of Curves: | "Airway Pressures vs Time ", Flow vs time,"Volume vs time ",CO2 vs Time |
| Capnography: | End-tidal CO2-conc. (etCO2), Minute elimination of CO2, Tidal CO2 elimination (VTCO2), |
| Trends: | MV, TV, RR, PEEP, R, C, etCO2, Peak-, plateau & mean pressures" |
| Loops: | Paw vs Vol, Flow vs Vol. |
| Oxygen Saturation (SpO2): | SpO2, Pulse, |
| You choose standard settings or "Paw-Manom, Loops, Big curves or Local" | |
ADU Carestation Ventilator
Normal settings in the respirator| Respiratory physiology parameters | Normal settings |
|---|---|
| TU | 5-15 cm H2O |
| TD | 6-8 ml/kg |
| AF | 10-20 breaths/minute |
| TU | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP | 5-15 cm H2O |
| FiO2 | 25-40% |
| ASB | 3-12 cm H2O |
| CPAP | 3-10 cm H2O |
Evita XL, Evita 4, Pulmovista (Dräger Ventilators)
Evita XL, Evita 4, Pulmovista
Dräger ventilators offer multiple breathing modes, such as IPPV, BiPaP, BiPaP-assist, CPAP + ASB, SIMV, APRV, and MMV. These modes provide various types of assisted or controlled ventilation via a mask or endotracheal tube. The ventilators feature advanced breathing modes like the “open breathing system” in AutoFlow, APRV, and integrated graphical support tools in the display as standard. Airway pressures are typically set for CPAP breaths and assisted breaths (ASB) with a normal pressure of 5-12 cm H2O.
| Dräger Evita Ventilator | Display Settings |
|---|---|
| Pulmonary Airway Pressure (Paw): | Peak Pressure, "Plateau Pressure ", Airway mean pressure , PEEP |
| Minute volume (MV): | "Inspiratory MV ", Expired MV, Spontaneous MV, MV leakage |
| Tidal Volume (VT): | Inspired TV,Expired TV, Assisted Spontaneuos TV (VTASB) |
| Respiratory Rate (RR): | " RR ", "Spontaneous RR ", Obligatory RR, |
| Breathing Work (WOB): | WOB in patient & ventilator, Shallow breathing index (SBI) |
| O2-concentration: | Inspiratory O2- Fraction (FiO2) |
| Time Measures: | Inspiration-/Expiration (I:E), Inspiration-/tot. Respiratory time (TI/TTOT), Time constant (TC) |
| Lung Mecanics: | Respiratory restistance, "Compliance (C) static (in the respiratory system)", Dynamic elastanse (E) |
| Apnéa Time | |
| Air temperature: | 18-51°C |
| Configuration of Curves: | "Airway Pressures vs Time ", Flow vs Time , "Volume vs Time ", CO2 vs Time |
| Capnography: | End-tidal CO2-conc. (etCO2), Minute Elimination of CO2, Tidal CO2 Elimination (VTCO2) |
| Trends: | "MV, TV, RR, PEEPi, R, C, etCO2, Peak-, plateau & mean pressures" |
| Loops: | Paw vs Volume, Flow vs Volume |
| Oxygen Saturation (SpO2): | SpO2, Pulse |
| Dräger Evita Ventilators | Normal Settings |
|---|---|
| PS: | 5-15 cm H2O |
| TV: | 6-8 ml/kg |
| RR: | 5-20 breaths/minute |
| PS: | 5-15 cm H2O |
| I:E | 1:2 |
| PEEP: | 5-15 cm H2O |
| FiO2: | 25-40% |
| ASB: | 3-12 cm H2O |
| CPAP: | 3-10 cm H2O |
Disclaimer:
The content on AnesthGuide.com is intended for use by medical professionals and is based on practices and guidelines within the Swedish healthcare context.
While all articles are reviewed by experienced professionals, the information provided may not be error-free or universally applicable.
Users are advised to always apply their professional judgment and consult relevant local guidelines.
By using this site, you agree to our Terms of Use.