Author:
Kai Knudsen
Updated:
2 September, 2025
This chapter covers muscle relaxants in anesthesia, including types, mechanisms of action, dosing, monitoring, and reversal methods These agents are used to keep the patient completely still and to create the best possible conditions for surgery and ventilation.
Neuromuscular Blockade
Muscle-relaxing drugs provide neuromuscular blockade by acting at the neuromuscular junction in striated muscles, which control muscle strength and respiration. Muscle relaxants are used to facilitate surgery, particularly in general surgery such as laparoscopic surgery, abdominal surgery, orthopedic surgery, neurosurgery, thoracic surgery, or other surgeries where it is necessary for the patient to remain completely still or muscle relaxed. Neuromuscular blockade is also used to achieve airway control with good intubation conditions and to better ventilate patients with lung diseases.
Axons from nerve endings usually connect to muscle fibers via “neuromuscular junctions” in the motor endplate, where neurotransmitter substances are released from presynaptic vesicles and mediate neuromuscular innervation. Acetylcholine is released from these synapses, rapidly crossing the synapse and binding to postsynaptic acetylcholine receptors to trigger activity. Acetylcholine receptors are cholinergic receptors but can also be activated by nicotine and are therefore called nicotinic receptors – nAchR (nicotinic acetylcholine receptor). Nicotinic receptors are a form of ligand-gated ion channels that open a channel when activated. This affects the resting potential, making the next nerve cell more likely to depolarize and generate muscle contractions.
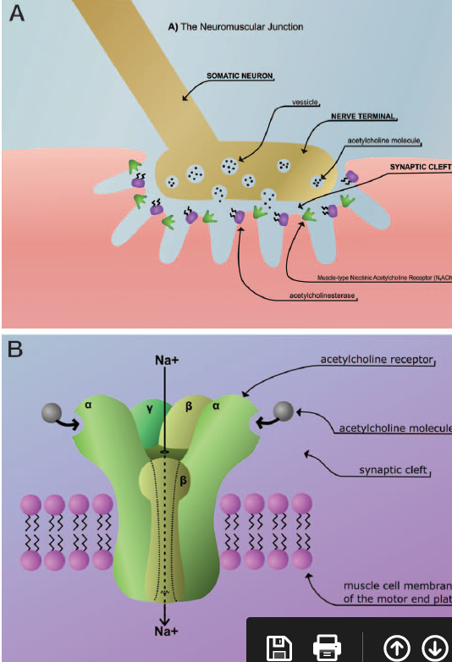
Muscle relaxants are usually quaternary ammonium compounds that structurally resemble acetylcholine. These bind to the alpha subunit of the nicotinic receptor and block them.
There are two types of muscle relaxants, depolarizing and non-depolarizing. Depolarizing muscle relaxants (succinylcholine) cause an initial stimulation that results in depolarization with muscle fasciculations, effectively discharging the muscles. The receptor is then closed to further nerve transmission until the muscle relaxant is broken down by the enzyme pseudocholinesterase. Non-depolarizing muscle relaxants bind to the nicotinic receptor and block nerve transmission, usually blocking muscle activity for at least 30 minutes after induction.
Succinylcholine is chemically structured like a double acetylcholine molecule. Upon administration, initial fasciculations are usually first visible around the eyes, immediately followed by the face and the rest of the body. Succinylcholine is fast-acting with a short duration, typically lasting 5-10 minutes. Succinylcholine provides the best intubation conditions and airway control. It is used not only for intubation but also to resolve laryngospasm and severe chest wall rigidity, such as after an overdose of remifentanil. The short duration is due to redistribution from neuromuscular junctions; the substance is broken down by pseudocholinesterase, which is the same as butyrylcholinesterase and plasma cholinesterase. There is a familial deficiency of pseudocholinesterase, which can cause some patients to experience prolonged muscle relaxation from succinylcholine. Usually, however, the effect subsides even in these patients after a few hours; the condition is rare. In such cases, the administration of succinylcholine should naturally be avoided. Prolonged effects of succinylcholine can occur with liver disease, cancer, and poor general health, and the use of other drugs metabolized by cholinesterase.

Succinylcholine has certain side effects, such as potassium release (caution with burns), arrhythmias, bradycardia (small children), postoperative muscle pain, malignant hyperthermia, and histamine release. It should not be given to patients after prolonged immobilization (long stay in ICU!), muscle diseases, burns (after 24 hours), spinal cord injuries, plasma cholinesterase deficiency, malignant hyperthermia, increased intracranial pressure, or uremia. However, in each individual case, the risks must be weighed against the benefits. Establishing a clear airway always has the highest priority in unconscious or semi-conscious patients, and succinylcholine is the fastest and most effective for good airway control.
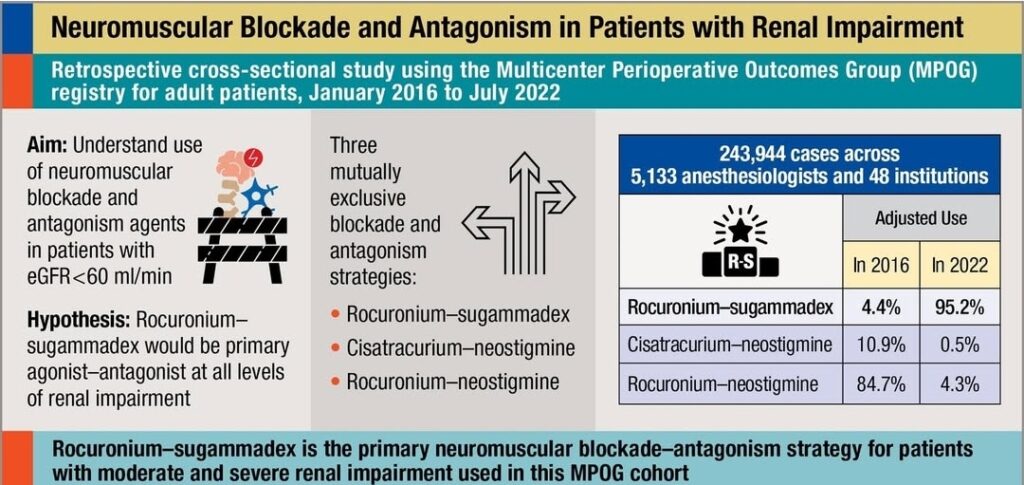
Non-depolarizing muscle relaxants are chemically structured as either steroid-based or benzylisoquinoline-based esters. Steroid-based non-depolarizing muscle relaxants include vecuronium and rocuronium, which are relatively similar. The steroid-based agents have a low frequency of allergic reactions, do not cross the placenta, and are metabolized by hydroxylation in the liver. Rocuronium is an intermediate-acting agent with active metabolites, which means some risk of prolonged effect in cases of liver failure. It is metabolized in the liver and excreted via bile. Rocuronium is an alternative to succinylcholine in rapid sequence induction (RSI) at higher than normal doses, 0.9-1.0 mg/kg. If necessary, the effect can be reversed with a specific antidote, sugammadex (Bridion).
Dosage of Muscle Relaxants
| Drug | Brand Name | Intubation Dose mg/kg | Repeated Dose = ED95 mg/kg |
|---|---|---|---|
| Suxamethonium (Succinylcholine) | Celocurin | 1-1,5 | |
| Mivacurium | Mivacron | 0,2-0,25 | 0,04-0,08 |
| Atracurium | Atracurium | 0,5-0,6 | 0,2-0,3 |
| Vecuronium | Norcuron | 0,08-0,1 | 0,03 |
| Rocuronium | Esmeron | 0,6-0,9 | 0,2-0,3 |
| Cisatracurium | Nimbex | 0,15-0,2 | 0,06-0,08 |
| Pancuronium | Pavulon | 0,08-0,1 | 0,03 |
Pancuronium is an older, long-acting agent with relatively high muscarinic activity that is rarely used today. Pancuronium was the standard drug for anesthetic muscle relaxation until the 1990s.
There are several other non-depolarizing agents pharmacologically categorized as benzylisoquinolines. These agents have relatively low muscarinic affinity. Notable among these are atracurium, cisatracurium, and mivacurium. These agents have a relatively high histamine-releasing effect, which clinically risks causing skin redness and bronchoconstriction. Atracurium and cisatracurium are broken down by non-specific plasma esterases and undergo spontaneous chemical breakdown via the so-called Hoffman elimination. Mivacurium is broken down by pseudocholinesterase. Elimination is independent of liver and kidney function.
Atracurium is an intermediate-acting non-depolarizing agent with a heterogeneous composition of isomers. Atracurium releases histamine and is eliminated via Hoffman elimination.
Mivacurium is a short-acting non-depolarizing muscle relaxant. It causes some histamine release and is broken down by pseudocholinesterase.
Reversal of Neuromuscular Blockade
The effect of non-depolarizing muscle relaxants is usually reversed with neostigmine, a cholinesterase inhibitor, restoring muscular innervation. Neostigmine can cause bradycardia and bronchoconstriction, so atropine is usually administered as an anticholinergic at the same time. A typical reversal dose for an adult is 2.5 mg neostigmine (1 ml) and 0.5 mg atropine (1 ml), totaling 2 ml. Neostigmine can increase acetylcholine levels in the synapse and displace non-depolarizing muscle relaxants. If the reversal is incomplete, an additional half dose can be administered (1/2 ml neostigmine + 1/2 ml atropine). The dosage of neostigmine is 30-70 µg/kg, but it is usually standardized to 1 ml (2.5 mg) for adults and adjusted according to weight for children. The effect of muscle relaxants and reversal should be continuously monitored using specific monitoring systems.
Neostigmine
- Dose: 30–70 μg/kg, with glycopyrronium or atropine
- Onset: ~10 min for full diaphragm recovery
- Ceiling effect: higher doses give no extra benefit
- Side effects: ↑ salivation, bronchoconstriction, ↑ GI motility, bradycardia, hypotension, nausea
- Acts on muscarinic + nicotinic receptors
Monitoring before extubation
- Use TOF (train-of-four) via n. ulnaris
- Safe extubation: TOF ≥ 90%
- TOF < 20% → use sugammadex
- TOF < 2 → do NOT use neostigmine (risk of recurarization)
Sugammadex (Bridion)
- Mechanism: encapsulates rocuronium/vecuronium, renal excretion
- Contraindication: severe renal failure
- Dosage:
- TOF ≥ 2: 2 mg/kg
- TOF < 2: 4 mg/kg
- Immediate reversal after rocuronium: 16 mg/kg
- Onset: 1.5–2 min depending on dose
- Give as IV bolus (10 sec)
- Avoid re-administering rocuronium/vecuronium within 24h after 16 mg/kg dose
Clinical risks
- Incomplete reversal → ↑ risk of hypoxia, aspiration, pulmonary complications, morbidity & mortality
- NMT monitoring: may interfere with pacemakers → caution required
Effect duration of muscle relaxants
| Drug | Brand name | Time until intubation (min) | Clinical Effect (min) |
|---|---|---|---|
| Suxamethonium (Succinylcholine) | Celocurin | < 1 | 5-10 |
| Mivacurium | Mivacron | 3-4 | 15-30 |
| Atracurium | Atracurium | 2-3 | 30-60 |
| Vecuronium | Norcuron | 2-3 | 30-60 |
| Rocuronium | Esmeron | 1-2 | 30-60 |
| Cisatracurium | Nimbex | 3-4 | 45-75 |
| Pancuronium | Pavulon | 2-3 | 45-180 |
Monitoring of neuromuscular blockade “TOF-Watch”
The effect of muscle relaxants on neuromuscular transmission (NMT) should always be continuously monitored using specific monitoring systems.
Conventional TOF systems use acceleromyography (AMG), a technology that measures the degree of muscle relaxation by recording how the thumb moves during neurostimulation. These systems are usually referred to simply as “TOF meters” or “TOF-watch”.
Best practice for reliable NMT monitoring is to stimulate the adductor pollicis muscle, which controls the thumb in the hand, by nerve stimulation of the ulnar nerve. This is done by sending a low-voltage current through two flat skin electrodes just proximal to the wrist, volar, and ulnar, and measuring the response in the form of twitches in the thumb, which is measured with a small sensor. The muscle twitches produce bars T1-T4, with a maximum of four. The skin electrodes are placed 2.5-4 cm apart. The sensor on the thumb is taped or mounted as a small splint. It is important to place the meter correctly (TOF meter), place the transducer correctly, ensure free mobility of the thumb, maintain a sufficient distance between the thumb and hand, and ensure the skin temperature is above 32 degrees. It becomes difficult to measure if the thumb is adducted against the hand. Neuromuscular activity is generally reduced in hypothermia. Stimulation and control of muscle strength are done through a varied pattern of electrical stimulation; Train-Of-Four (TOF), Single Twitch (Twitch), Post Tetanic Count (PTC), Double-Burst stimulation (DBS), or tetanus stimulation.
Train-Of-Four (TOF)
Neuromuscular transmission is measured by delivering four short electrical shocks to a peripheral nerve. The response is measured as four bars, “Train Of Four” (TOF). The responses are displayed on a separate monitor (TOF-Watch) or integrated into the existing anesthesia monitoring monitor. The first bar’s amplitude, the degree of amplitude reduction over four beats (TOF ratio), is interpreted as the amplitude of T4 through the amplitude of T1 (T4/T1). If fewer than four bars are detected, the monitor displays a TOF count value (TOFcnt) instead of a percentage (1-4). In complete neuromuscular blockade, no bars are seen in the TOF response. In moderate blockade that allows surgery, 1 to 4 bars are seen. During reversal, four bars are first returned. The original amplitude of the monitor bars in percentage, TOF% (TOFrat), is then recovered. As a guideline, extubation can usually be performed at more than TOF 90%. For superficial surgery, usually, 2-4 bars are required, for invasive surgery 1-2 bars. For complete muscle blockade, no bars – no TOF response. The frequency of stimulation varies with the type of surgery and the length of the operation. Stimulation is used to prevent the patient from suddenly tensing, perhaps coughing, and making surgery difficult for the surgeon. TOF measurement can prevent hearing the classic comment from the surgeon: “The patient is tensing!”.
Post-Tetanic Count (PTC)
In PTC, stimulation is initiated with four current pulses every 500 milliseconds (2 Hz). If a muscle response is detected, the response is recorded as a TOF value. If no muscle response is detected, five seconds of tetanic stimulation with current pulses at 50 Hz is given, followed by a 3-second pause, followed by 20 single current pulses per second (1 Hz). Muscle response detection is only active during the first four single pulses and the last 20. During the last 20 current pulses, the PTC value increases with each detected muscle response. PTC can be used when muscle responses to TOF ratio are absent and before they return as the effect of muscle relaxants wears off. TOF ratio is mainly used to evaluate NMT.
Single Twitch (Twitch)
Measures the response to single electrical stimulations. The module sends a single pulse, measures the strength of the resulting contraction, and then calculates a contraction value as Twitch %. The response is given as a percentage of the strength of the reference contraction.
Double-Burst Stimulation (DBS)
The Double-Burst stimulation pattern consists of a sequence of 3 current pulses delivered every 20 milliseconds (50 Hz), followed by a 750-millisecond pause, followed by a sequence of 3 current pulses every 20 milliseconds (50 Hz). With each detected muscle response, the DBS count value (DBScnt) increases.
TOF measurement allows for proper dosing of muscle relaxants and avoids overdosing and underdosing. It is very difficult to assess the degree of muscle relaxation without monitoring. During awakening, it is important that the patient can not only squeeze a hand but also hold the hand closed with force or hold the head against the chest without immediately falling back. The strength of the respiratory muscles is usually maintained much longer than that of the skeletal muscles. The diaphragm is paralyzed before the thumb muscles during anesthesia, but the diaphragm regains its strength before the thumb. The larynx is paralyzed as quickly as the diaphragm but regains strength faster.
The best way to assess muscle strength is with neuromuscular monitoring and TOF measurement. If the patient is significantly muscle-relaxed upon awakening, the patient must be immediately re-sedated to avoid the discomfort of being muscle-relaxed. If larger doses of muscle relaxants have been administered than are reasonable to reverse, it is better to let the patient sleep on the operating table, in the recovery room, or the ICU and wait for awakening. Incomplete reversal is referred to as residual curarization or residual block (RNMB), which is a serious condition that can quickly become life-threatening; deaths have occurred. Residual curarization is common, unpleasant, and potentially dangerous. It increases the risk of postoperative respiratory events, early postoperative lung complications, and increases the risk of mortality and morbidity. The goal during awakening is for the patient to regain TOF > 90% before extubation, but sometimes the patient wakes up faster and clinically shows good strength even at TOF 70%. It is then possible to reverse, but muscle strength must be carefully monitored after extubation. Residual curarization reduces the hypoxic ventilatory response, i.e., it increases the risk of hypoxia. It also causes pharyngeal dysfunction and can cause upper airway obstruction. At TOF 80-90%, one can experience double vision, inability to bite down, reduced control of facial expression, subjective swallowing difficulties, and a feeling of drowning or suffocation.
Reversal of neuromuscular blocking agents (NMBA)
Acetylcholinesterase is an enzyme that rapidly breaks down acetylcholine into choline and acetate. If this enzyme is inhibited with cholinesterase inhibitors, acetylcholine levels in the synapse quickly increase, thereby restoring neuromuscular transmission and muscle strength. Acetylcholine competitively displaces non-depolarizing neuromuscular blockers. The most commonly used cholinesterase inhibitor is neostigmine. Neostigmine is usually administered together with an anticholinergic agent such as glycopyrronium (Robinul) or atropine. The typical dose of neostigmine is 30–70 μg/kg. Doses higher than those providing maximal effect generally do not yield additional benefit. Reversal usually takes some time; for full recovery of the diaphragm muscle, about 10 minutes are required.
Neostigmine can also cause significant side effects such as increased salivation, bronchoconstriction, increased gastrointestinal motility, bradycardia, hypotension, and nausea. Neostigmine affects both muscarinic and nicotinic receptors. The muscarinic effects are counteracted by simultaneous administration of an anticholinergic agent.
Before extubation, neuromuscular function should be assessed using n. ulnaris stimulation. A TOF ≥ 90% is considered acceptable for safe extubation.
- If TOF < 20%, reversal with sugammadex is recommended.
The dose depends on the depth of the block. Incomplete reversal is unpleasant for the patient and potentially dangerous, as it increases the risk of postoperative pulmonary complications, hypoxia, aspiration, and higher morbidity and mortality. When preparing for emergence with low TOF values, reversal with either neostigmine or sugammadex should be performed. However, if TOF < 2, neostigmine should not be used, as recurarization may occur. In such cases, sugammadex (Bridion) can be used for reversal. If TOF ≥ 2 responses are present, sugammadex should be given at 2 mg/kg.
If TOF < 2 responses, then 4 mg/kg is given. If immediate reversal is required after rocuronium administration, the dose is 16 mg/kg. At TOF ≥ 20%, neostigmine may be used for reversal. The full effect of neostigmine occurs after about 10 minutes. The dose depends on the depth of the block. In high-risk patients or high-risk situations, sugammadex should be considered regardless of TOF values.
An alternative is to administer sugammadex (Bridion) as an antidote to certain non-depolarizing agents. This antidote inactivates rocuronium or vecuronium by encapsulating the molecule, which is then excreted via the kidneys. Sugammadex can be administered at any time after administration of rocuronium or vecuronium. It is pharmacologically inert and eliminated renally. Contraindication: severe renal failure. Rocuronium or vecuronium should not be re-administered within 24 hours after sugammadex if the high dose of 16 mg/kg was given.
The dosage of sugammadex is 2 mg/kg if spontaneous return of T2 is observed. The effect occurs within 2 minutes. If stimulation with Post Tetanic Count (PTC) shows 1–2 twitches, a higher dose of 4 mg/kg is given. If reversal is needed immediately after a full intubating dose, 16 mg/kg can be administered, which restores muscle activity within about 1.5 minutes. Sugammadex can be given as a rapid bolus injection over 10 seconds.
NMT monitoring may interfere with implanted pacemakers and should be used with caution in patients with a pacemaker.
Butyrylcholinesterase = pseudocholinesterase
- Enzyme produced in the liver
- Found in plasma, erythrocytes, liver, pancreas, and intestine
- Physiological function unknown
- Breaks down various cholin esters, including acetylcholine, succinylcholine, cocaine,
- Can be used against nerve gas or organophosphate poisoning
- Experimental treatment for cocaine addiction
Pseudocholinesterase deficiency
- Liver disease
- Malnutrition
- Malignancy
- Heart failure
- Kidney failure
- Pregnancy
- Extensive burns
- Muscle blockade usually lasts for 1 hour
Inherited butyrylcholinesterase deficiency
- Autosomal recessive
- 1:2000 to 1:5000
- More common in certain populations – Persian Jews, Inuit, certain tribes in India and Turkey
- 5 different alleles
- Usually a single amino acid substitution – 1 or 2 amino acids
- Lower activity
- A decrease of more than 75% is required for clinical significance
When should we suspect butyrylcholinesterase deficiency?
- Celocurin or mivacurium has been used
- The patient appears unresponsive
- Does not breathe as expected
- Other causes such as medication mix-up, hypotension, hypoglycemia, hypothermia excluded
- TOF measurement shows muscle blockade
Diagnostics
- Measurement of plasma cholinesterase – spectrophotometry
- Dibucaine test – inhibits 80% of the normal variant’s activity
- Genetic investigation
- Performed in Stockholm and Malmö
- Sampling – Li-heparin tube with light green cap
- Can stand at room temperature for up to 4 hours
- Special handling instructions – downloaded from respective hospital
- The sample is sent with a referral to the C-lab, which handles it and sends it to Stockholm/Malmö
- AnOpIVA is responsible for the costs
Alternative anesthesia method for butyrylcholinesterase deficiency
- Induction with propofol and remifentanil
- Esmeron as muscle relaxant 0.3 mg/kg (according to the literature)
- Intubation
- TOF measurement – TOF under 25% adequate
Neuromuscular Blocking Agents (NMBA)
Atracurium (Atracurium-hameln®)
Non-depolarizing muscle-relaxing drug. Adjuvant to general anesthesia to facilitate endotracheal intubation and to achieve relaxation of skeletal muscles during surgical procedures of medium to long duration. Patients are usually muscle relaxed during abdominal surgery (open or laparoscopic), orthopedic surgery, and other surgeries where the patient must remain absolutely still. To facilitate manual or mechanical ventilation of an anesthetized patient.
Concentration: 10 mg/ml solution
Intubation dose: 0.6 mg/kg iv (1.0 mg/kg in RSI); 40-60 mg to normal weight adult = 4-6 ml, (formerly Tracrium). Time to full effect about 90 seconds with adequate muscle relaxation for about 35 minutes.
Maintenance dose: 0.1-0.2 mg/kg (10-20 mg) every 30 minutes.
Standard dose: 50 mg iv. Maintenance 0.15 mg/kg 10-20 mg per occasion. Administered every 20 to 60 minutes during anesthesia. Spontaneous recovery after about 35 minutes.
Cave: Allergic reactions. Previous reaction to muscle relaxants, myasthenia, or similar neuromuscular disease. Should not be given i.m.
Reversal:
- Robinul-Neostigmine 1-2 ml intravenously (neostigmine 2.5-5 mg + glycopyrronium 0.5-1 mg) or
- Atropine 1 mg + Neostigmine 2.5 mg iv.
Suxamethonium (Celocurin®)
Depolarizing muscle relaxants. Ultra-short-acting agent with very rapid onset. Used to quickly achieve muscle relaxation and airway control. For intravenous use only. Adjuvant to general anesthesia to facilitate endotracheal intubation and to achieve relaxation of skeletal muscles during surgical procedures of short duration. To facilitate manual ventilation of an anesthetized patient. Onset within 60-90 seconds. Used to create optimal intubation conditions quickly.
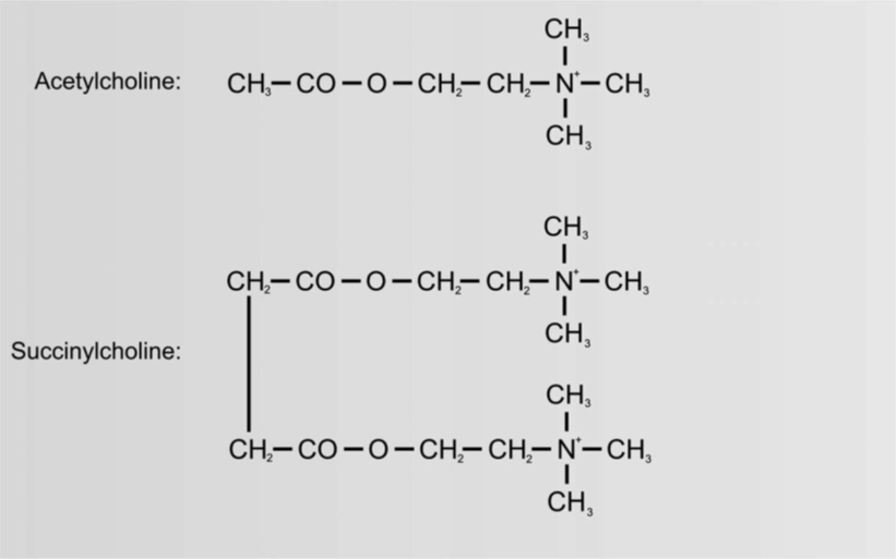
- Succinylcholine
- Binds to the nicotinic ACh receptor in the endplate
- Not metabolized by acetylcholinesterase AChE
- Succinylcholine diffuses away to the extracellular space and is broken down by butyrylcholinesterase
- BChE is present in plasma but not in the endplate
- ED95 – 0.3 mg/kg
- Only 10% of the given IV dose reaches the muscle endplate
- 90% is broken down by butyrylcholinesterase within 60 seconds
- 90% recovery 9-13 min after 1 mg/kg
- The short duration is due to butyrylcholinesterase and its activity
Concentration: 50 mg/ml solution.
Intubation dose: 1-1.5 mg/kg iv = 50-100 mg iv (possibly pretreat with atropine),
Standard dose: 75 mg iv, 50-100 mg, 1-2 ml iv.
Cave: Hyperkalemia, recent burns, malignant hyperthermia, muscle diseases, extensive tissue damage, bradycardia.
Reversal: none
Rocuronium (Esmeron®)
Non-depolarizing muscle-relaxing drug. Adjuvant to general anesthesia to facilitate endotracheal intubation and to achieve relaxation of skeletal muscles during surgical procedures of medium to long duration. Patients are usually muscle relaxed during abdominal surgery (open or laparoscopic), orthopedic surgery, and other surgeries where the patient must remain absolutely still. To facilitate manual or mechanical ventilation of an anesthetized patient.
Concentration: 10 mg/ml
Intubation dose
- 0.6 mg/kg iv (90 sec -> intub)
- 1.0 mg/kg (60 sec -> intub) 1.0 mg/kg in RSI
- 40-50 mg to normal weight adult = 4-5 ml
Standard dose: 50 mg. Maintenance 0.15 mg/kg, 10-20 mg per occasion. Administered every 20 to 60 minutes during general anesthesia.
Cave: Previous reaction to muscle relaxants, myasthenia gravis, or similar neuromuscular disease.
Reversal:
- Robinul-Neostigmine 1-2 ml intravenously (neostigmine 2.5-5 mg + glycopyrronium 0.5-1 mg) or
- Atropine 1 mg + Neostigmine 2.5 mg iv.
- Emergency reversal: Bridion 16 mg/kg (70 kg = 1120 mg = 11.2 ml). Administered iv as a bolus in 10 seconds
Mivacurium (Mivacron®)
Non-depolarizing muscle-relaxing drug. Adjuvant to general anesthesia to facilitate tracheal intubation and to achieve relaxation of skeletal muscles during surgical procedures of medium to long duration. Patients are usually muscle relaxed during abdominal surgery (open or laparoscopic), orthopedic surgery, and other surgeries where the patient must remain absolutely still.
Concentration: 2 mg/ml solution
Intubation dose: 0.07-0.25 mg/kg iv, 5-20 mg to normal weight adult
Standard dose: 15 mg iv. Maintenance 0.1 mg/kg (7.5 mg) per occasion. Administered every 20 to 60 minutes during general anesthesia.
Cave: Previous reaction to muscle relaxants, myasthenia, or similar neuromuscular disease.
Reversal:
- Robinul-Neostigmine 1-2 ml intravenously (neostigmine 2.5-5 mg + glycopyrronium 0.5-1 mg) or
- Atropine 1 mg + Neostigmine 2.5 mg iv.
Vecuronium (Norcuron®)
Non-depolarizing muscle-relaxing drug. Adjuvant to general anesthesia to facilitate endotracheal intubation and to achieve relaxation of skeletal muscles during surgical procedures of medium to long duration. Patients are usually muscle relaxed during abdominal surgery (open or laparoscopic), orthopedic surgery, and other surgeries where the patient must remain absolutely still. To facilitate manual or mechanical ventilation of an anesthetized patient.
Concentration: 2 mg/ml solution
Intubation dose: 0.08-0.1 mg/kg iv, 8-12 mg to normal weight adult = 4-6 ml.
Standard dose: 10 mg iv. Maintenance 0.02 to 0.03 mg/kg, 1-3 mg per occasion. Administered every 20 to 60 minutes during anesthesia.
Cave: Previous reaction to muscle relaxants, myasthenia gravis, or similar neuromuscular disease.
Reversal:
- Robinul-Neostigmine 1-2 ml intravenously (neostigmine 2.5-5 mg + glycopyrronium 0.5-1 mg) or
- Atropine 1 mg + Neostigmine 2.5 mg iv.
Pancuronium (Pavulon) – licensed product
Non-depolarizing muscle-relaxing drug. Previously standard but no longer available. Adjuvant to general anesthesia to facilitate endotracheal intubation and to achieve relaxation of skeletal muscles during surgical procedures of medium to long duration. Patients are usually muscle relaxed during abdominal surgery (open or laparoscopic), orthopedic surgery, and other surgeries where the patient must remain absolutely still. To facilitate manual or mechanical ventilation of an anesthetized patient.
Concentration: 2 mg/ml solution
Intubation dose: 0.08-0.1 mg/kg iv, 8-12 mg to normal weight adult = 4-6 ml
Standard dose: 10 mg iv. Maintenance 0.02 to 0.03 mg/kg, 1-3 mg per occasion. Administered every 20 to 60 minutes during anesthesia.
Cave: Previous reaction to muscle relaxants, myasthenia gravis, or similar neuromuscular disease.
Reversal:
- Robinul-Neostigmine 1-2 ml intravenously (neostigmine 2.5-5 mg + glycopyrronium 0.5-1 mg) or
- Atropine 1 mg + Neostigmine 2.5 mg iv.
Neostigmine
Neostigmine is a cholinesterase inhibitor that increases acetylcholine concentration, which reverses the effect of muscle-relaxing agents, allowing muscle activity and strength to return after muscle relaxation during anesthesia. It is used to reverse the effects of non-depolarizing muscle relaxants at the end of anesthesia. Neostigmine can also be used to increase bowel motility in paralytic ileus and to increase muscle activity in myasthenia gravis. Administration of neostigmine is preceded by intravenous administration of an anticholinergic agent (atropine sulfate or glycopyrronium). Neostigmine is then administered at a dose of 0.5-2.5 mg (0.2-1 ml) intravenously. In exceptional cases, doses of up to 5 mg (2 ml) may be required. The injection should be given slowly and with consideration of the effect.
Concentration: 2.5 mg/ml iv
Dosing: 30-70 µg/kg.
Standard dose for reversal 2.5 mg = 1 ml. If the effect is insufficient, half the standard dose can be repeated (after 10-15 minutes) = 0.5 ml.
It is routinely combined with anticholinergics such as atropine or glycopyrronium to prevent bradycardia and bronchospasm.
- Neostigmine 2.5 mg + 0.5 mg glycopyrronium (Robinul) (1 ml)
- Neostigmine 2.5 mg (1 ml) + 1 mg atropine (2 ml)
Caution
When used concurrently with beta-blockers, bradycardia and hypotension may occur.
Sugammadex (Bridion®)
Antidote to non-depolarizing muscle relaxants, for intravenous use. Sugammadex is a derivative of gamma-cyclodextrin. Sugammadex forms a complex with the neuromuscular blocking agents rocuronium and vecuronium in plasma, thereby reducing the amount of muscle relaxant available to bind to the nicotinic receptor in the neuromuscular synapse.
Dosage: For normal reversal 2-4 mg/kg, (4 mg/kg gives 280 mg (2.8 ml) to a 70 kg patient). For children and adolescents, only 2 mg/kg is given, providing 80 mg (0.8 ml) to a 40 kg child.
Dosage instructions for sugammadex (Bridion)
| Weight (kg) | Moderate blockade when second twitch (T2) is displayed | Deep blockade 1-2 post tetanic counts (PTC) | Immediate reversal 3 minutes after administration of up to 1.0 mg/kg rocuronium |
|---|---|---|---|
| 2 mg/kg | 4 mg/kg | 16 mg/kg | |
| 10 | 0,2 ml | 0,4 ml | - |
| 20 | 0,4 ml | 0,8 ml | - |
| 30 | 0,6 ml | 1,2 ml | - |
| 40 | 0,8 ml | 1,6 ml | 6,4 ml |
| 50 | 1,0 ml | 2,0 ml | 8,0 ml |
| 60 | 1,2 ml | 2,4 ml | 9,6 ml |
| 70 | 1,4 ml | 2,8 ml | 11,2 ml |
| 80 | 1,6 ml | 3,2 ml | 12,8 ml |
| 90 | 1,8 ml | 3,6 ml | 14,4 ml |
| 100 | 2,0 ml | 4,0 ml | 16,0 ml |
| 110 | 2,2 ml | 4,4 ml | 17,6 ml |
| 120 | 2,4 ml | 4,8 ml | 19,2 ml |
| Bridion 100 mg/ml is supplied in 2 ml and 5 ml ampoules. | |||
| Store in the outer packaging at room temperature. Do not freeze. | |||
Indication: Reversal of the muscle-relaxing agents rocuronium (Esmeron®) and vecuronium (Norcuron®). Effect usually occurs within 2 minutes with nearly complete recovery of muscle strength.
Concentration: Solution 100 mg/ml (2 or 5 ml ampoules).
Side effects: Hypersensitivity, anesthesia complications such as too rapid restoration of muscle tone during surgery.
Warning: Risk of rapid restoration of muscle tone during surgery, which can complicate the surgical work. Anaphylactic reactions may occur.
Disclaimer:
The content on AnesthGuide.com is intended for use by medical professionals and is based on practices and guidelines within the Swedish healthcare context.
While all articles are reviewed by experienced professionals, the information provided may not be error-free or universally applicable.
Users are advised to always apply their professional judgment and consult relevant local guidelines.
By using this site, you agree to our Terms of Use.

